Chapter 8: Multiple Reactions
Topics
- Types of Multiple Reactions Selectivity and Yield
- Parallel Reactions
- Reactions in Series
- Algorithm for Complex Reactions
- Applications of Algorithm
Types of Multiple Reactions Selectivity and Yield
TopTypes of Multiple Reactions
Use molar flow rates and concentrations; DO NOT use conversion!
There are 4 classes of Multiple Reactions.
1. Parallel Reactions
\(\text{A} \xrightarrow{k_1} \text{B}\)
\(\text{A} \xrightarrow{k_2} \text{C}\)
2. Series Reactions\(\text{A} \xrightarrow{k_1} \text{B} \xrightarrow{k_2} \text{C}\)
3. Complex Reactions: Series and Parallel aspects combined\(\text{A} \xrightarrow{k_1} 2\text{B} + \text{C}\)
\(\text{A} + 2\text{C} \xrightarrow{k_2} 3\text{D}\)
4. Independent Reactions\(\text{A} \xrightarrow{k_1} \text{C}\)
\(\text{B} \xrightarrow{k_2} \text{D}\)
Independent reactions typically occur in the catalytic cracking of crude oil to form gasoline.
Selectivity and Yield
There are two types of selectivity and yield: Instantaneous and Overall.
| Instantaneous | Overall | |
|---|---|---|
| Selectivity | \(S_{DU} = \frac{r_D}{r_U}\) | \(\tilde{S}_{DU} = \frac{F_D}{F_U}\) |
| Yield | \(Y_D = \frac{r_D}{-r_A}\) | \(\tilde{Y}_D = \frac{F_D}{F_{A_0} - F_A}\) |
Example
\(\text{A} + \text{B} \xrightarrow{k_1} \text{D} \quad (\text{desired product}, r_D = k_1 C_A^2 C_B)\)
\(\text{A} + \text{B} \xrightarrow{k_2} \text{U} \quad (\text{undesired product}, r_U = k_2 C_A C_B)\)
\(S_{D/U} = \frac{r_D}{r_U} = \frac{k_1 C_A^2 C_B}{k_2 C_A C_B} = \frac{k_1}{k_2} C_A\)
To maximize the selectivity of \(D\) with respect to \(U\), run at high concentration of \(A\) and use PFR.
Parallel Reactions
Top\(\text{A} \xrightarrow{k_1} \text{D} \quad (\text{Desired}) \quad r_D = k_1 C_A^\alpha\)
\(\text{A} \xrightarrow{k_2} \text{U} \quad (\text{Undesired}) \quad r_U = k_2 C_A^\beta\)
The net rate of disappearance of A
\(r_A = r_D + r_U\)
Instantaneous selectivity\(S_{D/U} = \frac{r_D}{r_U} = \frac{k_1 C_A^\alpha}{k_2 C_A^\beta} = \frac{k_1}{k_2} C_A^{\alpha - \beta}\)
If α > β use high concentration of A. Use PFR.If α < β use low concentration of A. Use CSTR.
Series Reactions
TopExample: Series Reaction in a batch reactor
\(\text{A} \xrightarrow{k_1} \text{B} \xrightarrow{k_2} \text{C}\)
This series reaction could also be written as
Reaction (1)
\(\text{A} \xrightarrow{k_1} \text{B}\)
: -r1A=k1CAReaction (2)
\(\text{A} \xrightarrow{k_2} \text{C}\)
: -r2B=k2CB
Mole Balance on every species
|
Species A:Combined mole balance and rate law for a control volume batch reactor. |
\(\text{Batch Reactor} \quad V = V_0\) \(\frac{1}{V_0} \frac{dN_A}{dt} = r_A\) |
Net Rate of Reaction of A |
|
|---|---|
| \(r_A = r_{1A} + 0\) | |
Rate Law |
|
| \(r_{1A} = -k_1 A C_A\) | |
Relative Rates |
|
| \(r_{1B} = -r_{1A}\) | |
\(\frac{dC_A}{dt} = -k_1 C_A\) |
|
| Integrating with CA=CA0 at t=0 and then rearranging |
\(C_A = C_{A0} \exp(-k_1 t)\) |
Mole Balance |
|
|
Species B: |
\(\frac{dC_B}{dt} = r_B\) |
Net Rate of Reaction of B |
|
\(r_B = r_{B, \text{NET}} = r_{1B} + r_{2B}\) |
|
Rate Law |
|
\(r_{2B} = -k_2 C_B\) |
|
Relative Rates |
|
\(r_B = k_1 C_A - k_2 C_B\) |
|
\(\frac{dC_B}{dt} = k_1 C_{A0} \exp(-k_1 t) - k_2 C_B\) |
|
Combine |
|
\(\frac{dC_B}{dt} + k_2 C_B = k_1 C_{A0} \exp(-k_1 t)\) |
|
|
Using the integrating factor i.f.: |
\(\text{i.f.} = \exp \int k_2 \, dt = \exp(k_2 t)\) |
Evaluate |
|
|
\(\frac{d\left[C_B \exp(k_2 t)\right]}{dt} = k_1 C_{A0} \exp\left[(k_2 - k_1)t\right]\) |
|
|
at t = 0, CB = 0 |
\(C_B = \frac{k_1 C_{A0}}{k_2 - k_1} \left[\exp(-k_1 t) - \exp(-k_2 t)\right]\) |
|
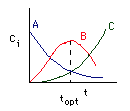
When should you stop the reaction to obtain the maximum amount of B? Let's see. |
|
Optimization of the Desired Product B |
|
\(t = t_{\text{opt}} \quad \text{at} \quad \frac{dC_B}{dt} = 0\) |
 |
|
Then |
\(t_{\text{opt}} = \frac{1}{k_2 - k_1} \ln\left(\frac{k_2}{k_1}\right)\) |
| Species C | \(C_C = C_{A0} - C_B - C_A\) |
|
And |
\(C_C = \frac{C_{A0}}{k_2 - k_1} \left[k_2\left(1 - e^{-k_1 t}\right) - k_1\left(1 - e^{-k_2 t}\right)\right]\) |
\(\text{A} \xrightarrow{k_1} \text{B} \xrightarrow{k_2} \text{C}\) in a CSTR
Schemes for maximizing the selectivity for Van Der Vusse Kinetics
\(\text{A} \rightleftharpoons \text{B} \xrightarrow{} \text{C}\)
\(2\text{A} \xrightarrow{} \text{D}\)
Can be found at the following web site http://www.wits.ac.za/centres/comps/AR/index.htm
Algorithm for Complex Reactions
Top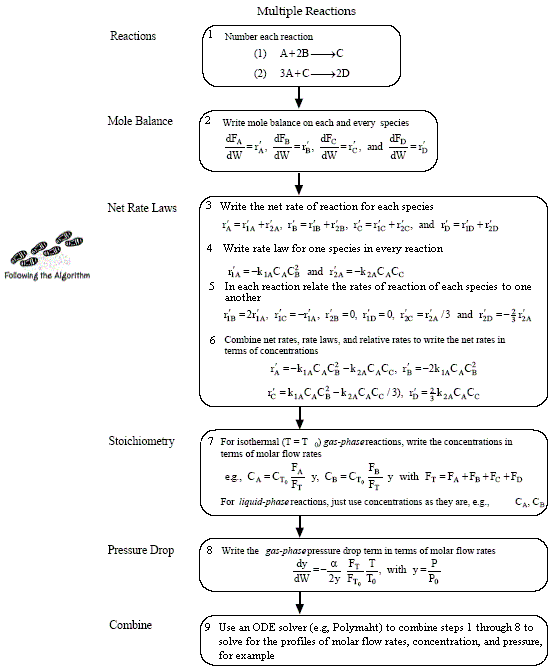
| Reactor Type | Gas Phase | Liquid Phase |
|---|---|---|
| Batch | \(\frac{dN_A}{dt} = r_A V\) | \(\frac{dC_A}{dt} = r_A\) |
| Semibatch | \(\frac{dN_A}{dt} = r_A V\) \(\frac{dN_B}{dt} = r_B V + F_{B0}\) |
\(\frac{dC_A}{dt} = r_A - \frac{u_0 C_A}{V}\) \(\frac{dC_B}{dt} = r_B + \frac{u_0 (C_{B0} - C_B)}{V}\) |
| CSTR | \(V = \frac{F_{A0} - F_A}{-r_A}\) | \(V = \frac{u_0 (C_{A0} - C_A)}{-r_A}\) |
| PFR | \(\frac{dF_A}{dV} = r_A\) | \(u_0 \frac{dC_A}{dV} = r_A\) |
| PBR | \(\frac{dF_A}{dW} = r'\) | \(u_0 \frac{dC_A}{dW} = r'\) |
Rates NOTE: The reaction rates in the above mole balances are net rates.
Stoichiometry
NOTE: We could use the gas phase mole balance for liquids and then just express the concentration as
Flow CA = FA/υ0
Batch CA =
NA/V0
\(c_i = c_{T0} \frac{F_i}{F_T} \frac{T_{0y}}{T}\)
\(F_T = \sum F_i = F_A + F_B + \ldots\)
Applications of Algorithm
Top| Reaction | Rate Equation | Note |
|---|---|---|
| \((1) \quad \text{A} + 2\text{B} \rightarrow \text{C}\) | \(-r_{1A} = k_{1A} C_A C_B^2\) | NOTE: The specific reaction rate \(k_{1A}\) is defined with respect to species A. |
| \((2) \quad 3\text{C} + 2\text{A} \rightarrow \text{D}\) | \(-r_{2C} = k_{2C} C_C^3 C_A^2\) | NOTE: The specific reaction rate \(k_{2C}\) is defined with respect to species C. |
These reactions will be used in the following 5 examples
• Liquid Phase PFR
• Liquid Phase CSTR
• Gas Phase PFR no ΔT
• Gas Phase Membrane Reactor with ΔT
• Liquid Phase Semibatch Reactor
Example A: Liquid Phase PFR
| (1) | \(\text{A} + 2\text{B} \rightarrow \text{C}\) | \(-r_{1A} = k_{1A} C_A C_B^2\) |
| (2) | \(2\text{A} + 3\text{C} \rightarrow \text{D}\) | \(-r_{2C} = k_{2C} C_A^2 C_C^3\) |
The complex liquid phase reactions follow elementary rate laws and take place in a PFR. The feed is equal molar in A and B with FA0 = 200 mol/min and the volumetric flow rate is 100 dm3/min. The reaction volume is 50 dm3 and the rate constants are
\(k_{1A} = 10 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}\)
\(k_{2C} = 15 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\)
Solution
Liquid PFR
Mole Balances
Equation Expression Additional Information (1) \(\frac{dF_A}{dV} = r_A\) \(F_{A0} = 200 \, \text{mol/min}\) (2) \(\frac{dF_B}{dV} = r_B\) \(F_{B0} = 200 \, \text{mol/min}\) (3) \(\frac{dF_C}{dV} = r_C\) \(V_F = 50 \, \text{dm}^3\) (4) \(\frac{dF_D}{dV} = r_D\) Net Rates
Equation Expression (5) \(r_A = r_{1A} + r_{2A}\) (6) \(r_B = r_{1B}\) (7) \(r_C = r_{1C} + r_{2C}\) (8) \(r_D = r_{2D}\) Rate Laws
Equation Expression (9) \(r_{1A} = -k_{1A} C_A C_B^2\) (10) \(r_{2C} = -k_{2C} C_A^2 C_C^3\) Relative Rates
Equation Expression Reaction \(\frac{r_{1A}}{-1} = \frac{r_{1B}}{-2} = \frac{r_{1C}}{1}\) Reaction 1 (11) \(r_{1B} = 2 r_{1A}\) (12) \(r_{1C} = -r_{1A}\) \(\frac{r_{2A}}{-2} = \frac{r_{2C}}{-3} = \frac{r_{2D}}{1}\) Reaction 2 (13) \(r_{2A} = \frac{2}{3} r_{2C}\) (14) \(r_{2D} = -\frac{1}{3} r_{2C}\) Selectivity
If one were to write SC/D = FC/FD in the Polymath program, Polymath would not execute because at V = 0, FC = 0 resulting in an undefined volume (infinity) at V = 0. To get around this problem we start the calculation 10-4 dm3 from the reactor entrance where FD will note be zero and use the following IF statement.
Equation Expression (15) \(\tilde{S}_{C/D} = \text{if } (V > 0.001) \text{ then } \left(\frac{F_C}{F_D}\right) \text{ else } (0)\)
Stoichiometry
Equation Expression (16) \(C_A = \frac{F_A}{\nu_0}\) (17) \(C_B = \frac{F_B}{\nu_0}\) (18) \(C_C = \frac{F_C}{\nu_0}\) (19) \(C_D = \frac{F_D}{\nu_0}\)
Parameters
Equation Expression (20) \(\nu_0 = 100 \, \text{dm}^3/\text{min}\) (21) \(k_{1A} = 10 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}\) (22) \(k_{2C} = 15 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\)
Would you like to see the Polymath results for Example A: Multiple Reactions, PFR, Liquid Phase
Would you like to run Polymath for Example A: Multiple Reactions, PFR, Liquid Phase
Example B: Liquid Phase CSTR
Same reactions, rate laws, and rate constants as example A
(1) \(\text{A} + 2\text{B} \rightarrow \text{C}\) \(-r_{1A} = k_{1A} C_A C_B^2\) (2) \(2\text{A} + 3\text{C} \rightarrow \text{D}\) \(-r_{2C} = k_{2C} C_A^2 C_C^3\)
The complex liquid phase reactions take place in a 2,500 dm3 CSTR. The feed is equal molar in A and B with FA0 = 200 mol/min, the volumetric flow rate is 100 dm3/min and the reaction volume is 50 dm3.
Find the concentrations of A, B, C, and D exiting the reactor along with the exiting selectivity.
Plot FA, FB, FC, FD and SC/D as a function of VSolution
Liquid CSTR
Mole Balances
Equation Expression (1) \(f(C_A) = \nu_0 C_{A0} - \nu_0 C_A + r_A V\) (2) \(f(C_B) = \nu_0 C_{B0} - \nu_0 C_B + r_B V\) (3) \(f(C_C) = -\nu_0 C_C + r_C V\) (4) \(f(C_D) = -\nu_0 C_D + r_D V\) Net Rates
Equation Expression (5) \(r_A = r_{1A} + r_{2A}\) (6) \(r_B = r_{1B}\) (7) \(r_C = r_{1C} + r_{2C}\) (8) \(r_D = r_{2D}\) Rate Laws
Equation Expression (9) \(r_{1A} = -k_{1A} C_A C_B^2\) Relative Rates
Equation Expression Reaction (10) \(r_{2C} = -k_{2C} C_A^2 C_C^3\) \(\frac{r_{1A}}{-1} = \frac{r_{1B}}{-2} = \frac{r_{1C}}{1}\) Reaction 1 (11) \(r_{1B} = 2 r_{1A}\) (12) \(r_{1C} = -r_{1A}\) \(\frac{r_{2A}}{-2} = \frac{r_{2C}}{-3} = \frac{r_{2D}}{1}\) Reaction 2 (13) \(r_{2A} = \frac{2}{3} r_{2C}\) (14) \(r_{2D} = -\frac{1}{3} r_{2C}\) Selectivity
Equation Expression (15) \(S_{C/D} = \frac{F_C}{F_D + 0.001}\) Parameters
Equation Expression (16) \(\nu_0 = 100 \, \text{dm}^3/\text{min}\) (17) \(k_{1A} = 10 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}\) (18) \(k_{2C} = 15 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\) (19) \(V = 2,500 \, \text{dm}^3\) (20) \(C_{A0} = 2.0\) (21) \(C_{B0} = 2.0\) Would you like to see the Polymath results for Example B: Multiple Reactions, CSTR, Liquid Phase
Would you like to run Polymath for Example B: Multiple Reactions, CSTR, Liquid Phase
Example C: Gas Phase PFR, No Pressure Drop
Same reactions and rate laws as previous two examples
(1) \(\text{A} + 2\text{B} \rightarrow \text{C}\) \(-r_{1A} = k_{1A} C_A C_B^2\) (2) \(2\text{A} + 3\text{C} \rightarrow \text{D}\) \(-r_{2C} = k_{2C} C_A^2 C_C^3\)
The complex gas phase reactions take place in a PFR. The feed is equal molar in A and B with FA0 = 10 mol/min and the volumetric flow rate is 100 dm3/min. The reactor volume is 1,000 dm3, there is no pressure drop, the total entering concentration is CT0 = 0.2 mol/dm3 and the rate constants are
\(k_{1A} = 100 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}\)
\(k_{2C} = 1,500 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\)
Plot \(F_A, F_B, F_C, F_D\) and \(\tilde{S}_{C/D}\) as a function of V
Solution
Gas Phase PFR, No Pressure Drop
Mole Balances
Equation Expression Additional Information (1) \(\frac{dF_A}{dV} = r_A\) \(F_{A0} = 10\) (2) \(\frac{dF_B}{dV} = r_B\) \(F_{B0} = 10\) (3) \(\frac{dF_C}{dV} = r_C\) \(V_f = 1,000\) (4) \(\frac{dF_D}{dV} = r_D\) Net Rates
Equation Expression (5) \(r_A = r_{1A} + r_{2A}\) (6) \(r_B = r_{1B}\) (7) \(r_C = r_{1C} + r_{2C}\) (8) \(r_D = r_{2D}\) Rate Laws
Equation Expression (9) \(r_{1A} = -k_{1A} C_A C_B^2\) (10) \(r_{2C} = -k_{2C} C_A^2 C_C^3\) Relative Rates
Equation Expression Reaction \(\frac{r_{1A}}{-1} = \frac{r_{1B}}{-2} = \frac{r_{1C}}{1}\) Reaction 1 (11) \(r_{1B} = 2 r_{1A}\) (12) \(r_{1C} = -r_{1A}\) \(\frac{r_{2A}}{-2} = \frac{r_{2C}}{-3} = \frac{r_{2D}}{1}\) Reaction 2 (13) \(r_{2A} = \frac{2}{3} r_{2C}\) (14) \(r_{2D} = -\frac{1}{3} r_{2C}\) Selectivity
Equation Expression (15) \(S_{C/D} = \text{if } (V > 0.0001) \text{ then } \left(\frac{F_C}{F_D}\right) \text{ else } (0)\) Stoichiometry
Equation Expression (16) \(C_A = C_{T0} \left(\frac{F_A}{F_T}\right) y\) (17) \(C_B = C_{T0} \left(\frac{F_B}{F_T}\right) y\) (18) \(C_C = C_{T0} \left(\frac{F_C}{F_T}\right) y\) (19) \(C_D = C_{T0} \left(\frac{F_D}{F_T}\right) y\) (20) \(y = 1\) (21) \(F_T = F_A + F_B + F_C + F_D\) Parameters
Equation Expression (22) \(C_{T0} = 0.2 \, \text{mol}/\text{dm}^3\) (23) \(y = 1\) (24) \(k_{1A} = 100 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}\) (25) \(k_{2C} = 1,500 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\)
Would you like to see the Polymath results for Example C: Multiple Reactions, Gas Phase
Would you like to run Polymath for Example C: Multiple Reactions, Gas Phase
Example D: Membrane Reactor with Pressure Drop
Same reactions and rate laws as previous two examples
(1) \(\text{A} + 2\text{B} \rightarrow \text{C}\) \(-r_{1A} = k_{1A} C_A C_B^2\) (2) \(2\text{A} + 3\text{C} \rightarrow \text{D}\) \(-r_{2C} = k_{2C} C_A^2 C_C^3\)
The complex gas phase reactions take place in a catalytic packed bed with C diffusing out the sides. The feed is equal molar in A and B with FA0 = 10 mol/min and the volumetric flow rate is 100 3/min. The reactor volume is 50 dm3 and the total entering concentration is CT0 = 0.2 mol/dm3. There is pressure drop and entering pressure is 100 atm and the rate constants are
\(k_{1A} = 1,000 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}, \, k_{2C} = 60,000 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\)
The pressure drop parameter αρb = 0.0405 dm-3
\(R_C = k_{CC} \left[C_C - C_{Csg}\right]\)
The mass transfer coefficient for C is kcc = 2 min–1
Plot FA, FB, FC, FD and SC/D as a function of V for
(a) Case 1 CCsg = 0
(b) Case 2 CCsg ≠ 0,
\(C_{Csg} = \frac{F_{Csg}}{\upsilon_{sg}}, \, \upsilon_{sg} = \upsilon_{sgo} \left(\frac{F_{Osg} + F_{Csg}}{F_{Osg}}\right)\)
Set Fosg = 0.1 mol/min and vary \(\upsilon_{sgo}\)
(5 < \(\upsilon_{sgo}\) > < 10,000)
Are there a set of conditions whereby (CCsg < CC) and RC changes sign and Species C diffuses back into the membrane reactor near the exit? Run the Polymath program when αρb = 0 and compare RC with the base case when there IS pressure drop (αρb = 0.0405 dm-3)
Solution
Gas Phase Multiple Reactions in a Catalytic Packed Bed Membrane Reactor with Pressure Drop
Mole Balances
\(\frac{dF_A}{dV} = r_A\)
\(\frac{dF_B}{dV} = r_B\)
\(\frac{dF_C}{dV} = r_C - R_C\)
\(\frac{dF_D}{dV} = r_D\)
We also need to account for the molar rate desired product C leaving in the sweep gas FCsg
\(\frac{dF_{Csg}}{dV} = R_C\)
Rate Laws
- Net rates, rate laws and relative rates same as Liquid and Gas Phase PFR and Liquid Phase CSTR.
- Transport Law
\(R_C = k_{CC} \left[C_C - C_{Csg}\right]\)
Case 1 Large sweep gas velocity\(C_C \gg C_{Csg}\)
\(R_C = k_{CC} C_C\)
Case 2 Moderate to small sweep gas velocity\(R_C = k_{CC} \left[C_C - C_{Csg}\right]\)
\(C_{Csg} = \frac{F_{Csg}}{\upsilon_{sg}}\)
\(\upsilon_{sg} = \upsilon_{sgo} \left(\frac{F_{Osg} + F_{Csg}}{F_{Osg}}\right)\)
Vary \(\upsilon_{sg}\) to see changes in profiles
Case 2A Pressure Drop
Case 2B No Pressure DropStoichiometry
\(C_A = C_{T0} \frac{F_A}{F_T} y\)
\(C_B = C_{T0} \frac{F_B}{F_T} y\)
\(C_C = C_{T0} \frac{F_C}{F_T} y\)
\(C_D = C_{T0} \frac{F_D}{F_T} y\)
\(F_T = F_A + F_B + F_C + F_D\)
We need to reconsider our pressure drop equation when one or more species diffuse out of the reactor. Recall the pressure drop equation is
\(\frac{dy}{dW} = -\frac{\alpha}{2y} \frac{T}{T_0} \frac{F_T}{F_{T0}}\)
with
\(\alpha = \frac{2 \beta_0}{A_c (1 - \phi) \rho_c} \frac{1}{P_0}\)
\(\beta_0 = \frac{G}{\rho_0 g_c D_P} \left(\frac{1 - \phi}{\phi^3}\right) \left[\frac{150 (1 - \phi) \mu}{D_P} + 1.75 G\right]\)
Warning!!
When mass diffuses out of a membrane reactor as there will be a decrease in the superficial mass flow rate \(\dot{M}\) and hence G. To account for this decrease in calculating our pressure drop parameter , we will take the ratio of the superficial mass velocity at any point in the reactor to the superficial mass velocity at the entrance to the reactor. The superficial mass flow rates can be obtained by multiplying the species molar flow rates, Fi, by their respective molecular weights, MWi, and then summing over all species
\(\frac{G_2}{G_1} = \frac{m_2 / A_{C2}}{m_1 / A_{C1}} = \frac{\sum F_i (\text{MW}_i) / A_{C2}}{\sum F_{i0} (\text{MW}_i) / A_{C1}} = \frac{\sum F_i (\text{MW}_i) A_{C1}}{\sum F_{i0} (\text{MW}_i) A_{C2}}\)
Because the smallest molecule is the one diffusing out and has the lowest molecular weight, we will neglect the changes in the mass flow rate down the reactor and will take as a first approximation.
Isothermal (T = T0) and multiply both sides of the pressure drop equation by the bulk density, ρb
\(\rho_b \frac{dy}{dW} = -\frac{\alpha \rho_b F_T}{2y F_{T0}}\)
\(\frac{dy}{dW / \rho_b} = \frac{dy}{d(W / \rho_b)} = \frac{dy}{dV}\)
\(\boxed{\frac{dy}{dV} = -\frac{\alpha \rho_b F_T}{2y F_{T0}}}\)
\(\alpha \rho_b = 0.041 \, \text{dm}^{-3}\)
\(\frac{dy}{dV} = -\frac{\rho_b \alpha F_T}{2y F_{T0}}\)
Selectivity
Need to include C collected from sweep gas
\(S_{C/D} = \frac{F_C + F_{Csg}}{F_D}\)
\(S_{C/D} = \text{if } (V > 0.0001) \text{ then } \left(\frac{F_C + F_{Csg}}{F_D}\right) \text{ else } (0)\)
Parameters
\(k_{1A} = 1,000 \left(\frac{\text{dm}^3}{\text{mol}}\right)^2/\text{min}\)
\(k_{2C} = 60,000 \left(\frac{\text{dm}^3}{\text{mol}}\right)^4/\text{min}\)
\(k_C = 200 \, \text{min}^{-1}\)
\(F_{T0} = 20 \, \text{mol}/\text{min}\)
\(\rho_B \alpha = 0.0405 \, \text{dm}^{-3}\)
\(\upsilon_{Osg} = 5 \, \text{dm}^3/\text{min}\)
\(F_{Osg} = 0.1 \, \text{mol}/\text{min}\)
Would you like to see the Polymath results for Example D: Multiple Reactions, Membrane, Gas Phase, with Pressure Drop
Would you like to run Polymath for Example D: Multiple Reactions, Membrane, Gas Phase, with Pressure Drop
Example E: Liquid Semibatch
Same reactions, rate laws, and rate constants as example A
(1) \(\text{A} + 2\text{B} \rightarrow \text{C}\) \(-r_{1A} = k_{1A} C_A C_B^2\) (2) \(2\text{A} + 3\text{C} \rightarrow \text{D}\) \(-r_{2C} = k_{2C} C_A^2 C_C^3\)
The complex liquid phase reactions take place in a semibatch reactor where A is fed to B with FA0 = 3 mol/min. The volumetric flow rate is 10 dm3/min and the initial reactor volume is 1,000 dm3.
The maximum volume is 2,000 dm3 and CA0 = 0.3 mol/dm3 and CB0 = 0.2 mol/dm3. Plot CA, CB, CC, CD and SC/D as a function of time.
Solution
Liquid Phase Multiple Reactions in a Semibatch Reactor
Mole Balances
\(\frac{dN_A}{dt} = r_A V + F_{A0} \quad (N_{A0} = 0)\)
\(\frac{dN_B}{dt} = r_B V \quad (N_{B0} = C_{B0} V_0 = 2,000)\)
\(\frac{dN_C}{dt} = r_C V \quad (N_{C0} = 0)\)
\(\frac{dN_D}{dt} = r_D V \quad (N_{D0} = 0)\)
Net Rates, Rate Laws and relative rates – are the same as Liquid and Gas Phase PFR and Liquid Phase CSTR.
Stoichiometry
\(C_A = \frac{N_A}{V}\)
\(C_B = \frac{N_B}{V}\)
\(C_C = \frac{N_C}{V}\)
\(C_D = \frac{N_D}{V}\)
\(V = V_0 + \upsilon_0 t\)
Selectivity
\(S_{C/D} = \text{if } (t > 0.0001) \text{ then } \left(\frac{N_C}{N_D}\right) \text{ else } (0)\)
Parameters
\(\text{New Parameters}\)
\(\upsilon_0 = 10\)
\(V_0 = 1,000\)
\(F_{A0} = 3\)
Would you like to see the Polymath results for Example E: Semibatch, Liquid Phase
Would you like to run Polymath for Example E: Semibatch, Liquid Phase
Explore the cobra bites web module
* All chapter references are for the 1st Edition of the text Essentials of Chemical Reaction Engineering .
