Chapter 8: Multiple Reactions
Gas Phase Multiple Reactions in a Membrane Reactor with Pressure Drop
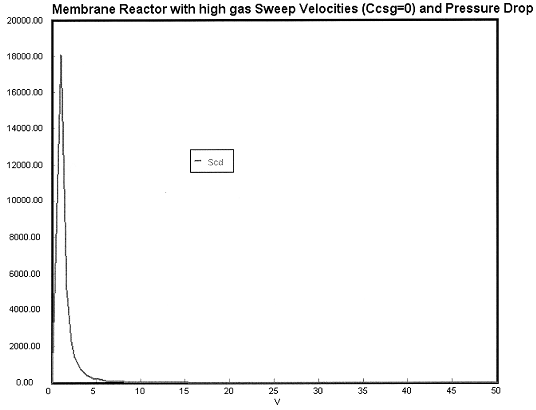
Case 1 Large sweep gas velocities CCsg = 0
Open the Polymath file for Case 1 by clicking here
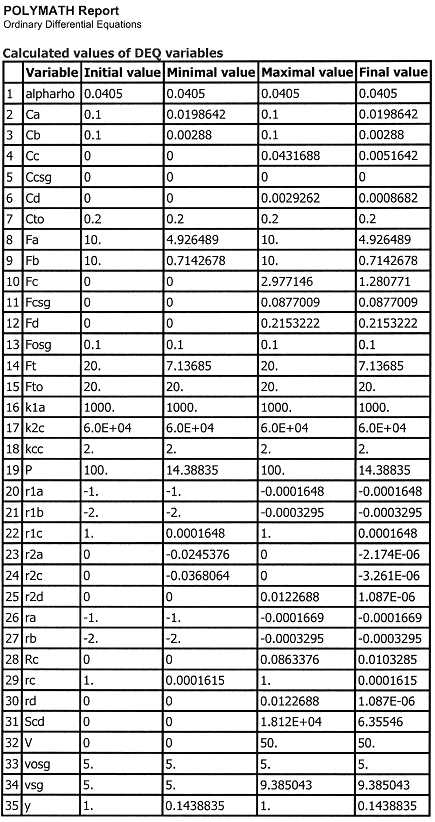

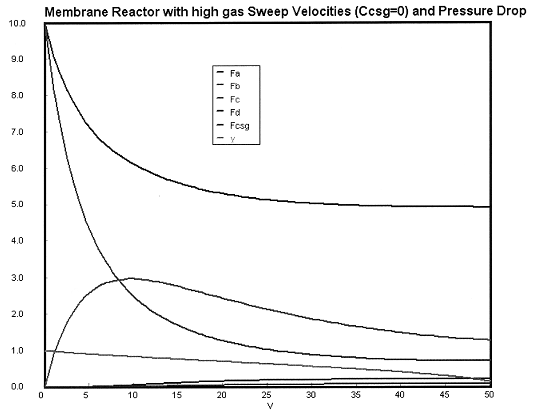
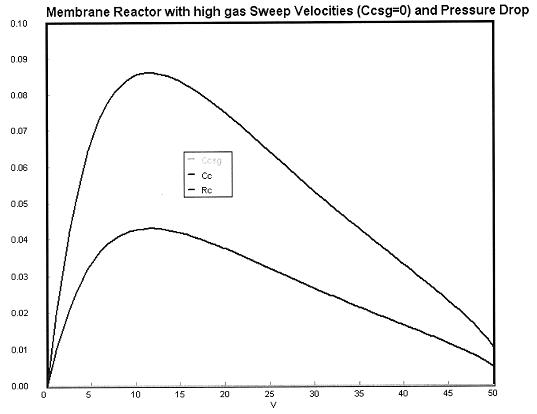
Case 2A Moderate to Low Sweep Gas Velocity CCsg = FCsg/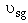
Open the Polymath file for Case 2 by clicking here
Entering sweep gas molar flow rate: \(F_{osg} = 0.1 \, \text{mol}/\text{min}\)
Entering sweep gas volumetric flow rate: \(\nu_{osg} = 5 \, \text{dm}^3/\text{min}, \, \nu_{sg} = 5 \left(\frac{0.1 + F_{csg}}{0.1}\right)\)
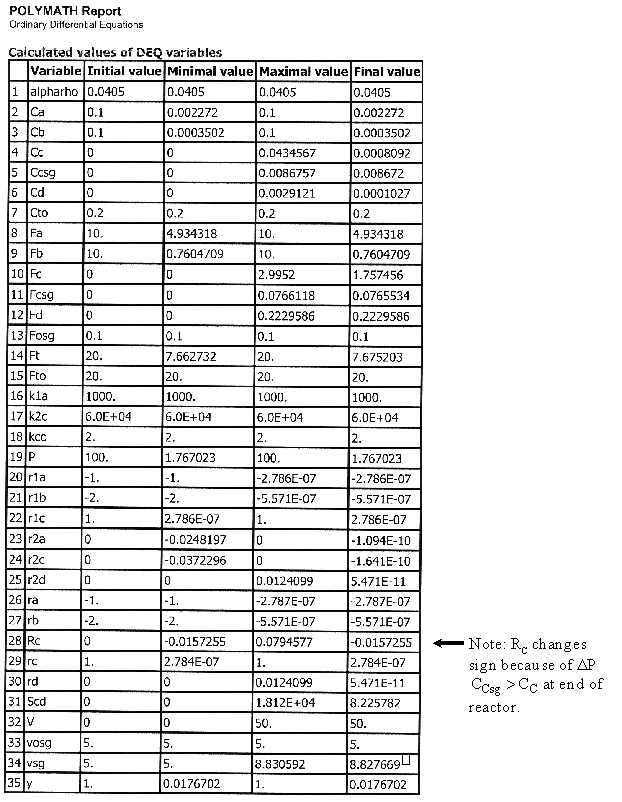
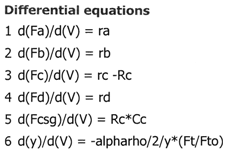

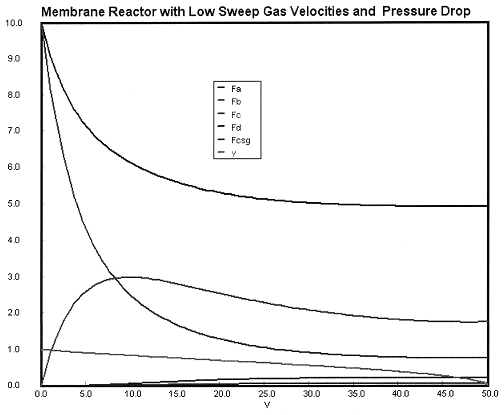
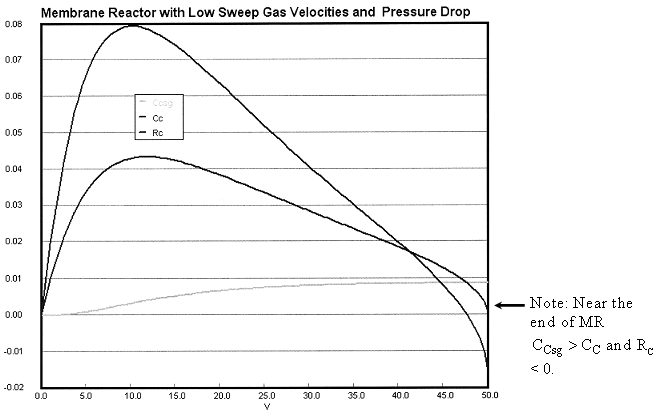
Case 2B Low Sweep Gas Velocity and No Pressure Drop
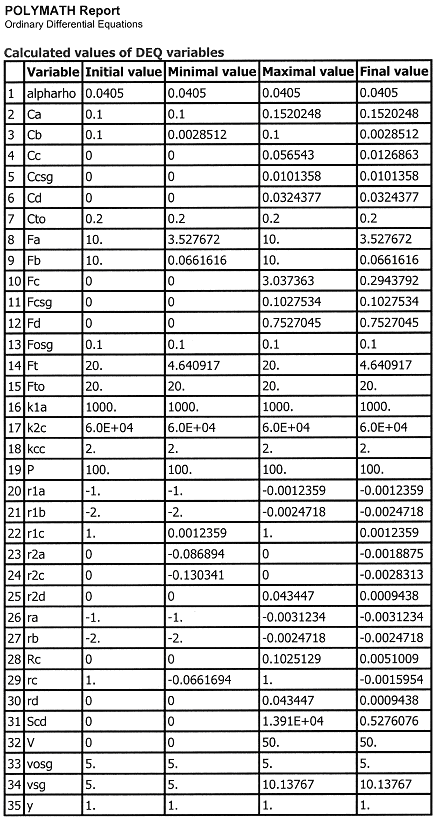
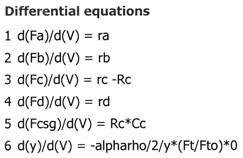

We see that when there is no pressure drop CC is always grater than CCsg and species C does not diffuse back into the reactor.
Back to Chapter 8