Chapter 8: Multiple Reactions
Maximizing the Selectivity - Parallel Reactions
Determine the instantaneous selectivity, SD/U, for the liquid phase reactions:
\( A + B \rightarrow D \quad \quad r_D = k_1 C_A^2 C_B \)
\( A + B \rightarrow U_1 \quad \quad r_{U_1} = k_2 C_A C_B \)
\( A + B \rightarrow U_2 \quad \quad r_{U_2} = k_3 C_A^3 C_B \)
Sketch the selectivity as a function of the concentration of A. Is there an optimum and if so what is it?
Hint 1: Write the equation for selectivity
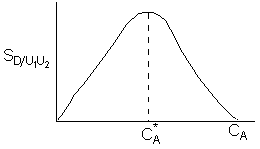
Hint 2: Sketch the selectivity as a funtion of CA
Full Solution: Find the optimum
|
Hint 1
\( S_{D/U_1U_2} = \frac{r_D}{r_{U_1} + r_{U_2}} = \frac{k_1 C_A^2 C_B}{k_2 C_A C_B + k_3 C_A^3 C_B} = \frac{k_1 C_A}{k_2 + k_3 C_A^2} \)
Hint 2
Determine the instantaneous selectivity, SD/U, for the liquid phase reactions:
\( A + B \rightarrow D \quad \quad r_D = k_1 C_A^2 C_B \)
\( A + B \rightarrow U_1 \quad \quad r_{U_1} = k_2 C_A C_B \)
\( A + B \rightarrow U_2 \quad \quad r_{U_2} = k_3 C_A^3 C_B \)
Sketch the selectivity as a function of the concentration of A. Is there an optimum and if so what is it?Solution
\( S_{D/U_1U_2} = \frac{r_D}{r_{U_1} + r_{U_2}} = \frac{k_1 C_A^2 C_B}{k_2 C_A C_B + k_3 C_A^3 C_B} = \frac{k_1 C_A}{k_2 + k_3 C_A^2} \)

Finding the optimum concentration:
\(\frac{dS}{dC_A} = 0 = k_1 \left[ k_2 + k_3 C_A^{*2} \right] - k_1 C_A^* \left[ 2k_3 C_A^* \right] \)
\( C_A^* = \sqrt{\frac{k_2}{k_3}} \)
Use CSTR with exit concentration C*A
Back to Chapter 8