 |
        |
|

|
|
Observed Stereochemistry Observed During Reaction of 12 to 13
During the reaction from compound 12 to 13, one can see a retention of stereochemistry in all the chiral centers that are not affected in a reaction. However, there is the addition of one additional chiral stereocenter in the form of a ketal.
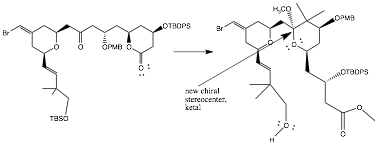
One explanation for the observed stereochemistry at this new chiral site is the retention of the axial bridge from the original, unaffected stereosite. Due to steric hindrance, the methanol compound in the following step would prefer to attack from the back side of the molecule to avoid collision with the carbon atom involved in the bridge. This bridge also may not be rotated due to its association with two rings.
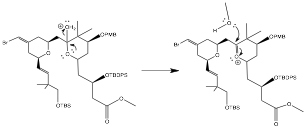
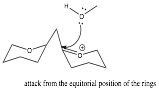
This reaction is SN-1 like due to the presence of an intermediate before the water molecule is replaced. This reaction is also driven by lone-pair assisted ionization.
Trost, B. M.; Guangbin, D. J. Am. Chem. Soc., 2010, 132, 16403-16416. 2010, 132, 371-383.
|
|
|