Compound 12 underwent an acid-catalyzed transformation during this experiment. Lactone 12 reacted with 18 mg (.080 mmole) CSA in dry MeOH (18 mL) at 0°C. Then, the mixture was stirred for 12 hours and poured into saturated NaHCO3. After extracting the mixture three times with ethyl acetate, the combined organics were dried with Na2SO4. The resulting alcohol 13 formed with ring A and B in a 93-96% yield and was purified using silica gel flash column chromatography. The product was 0.76 grams of colorless oil.
Glossary:
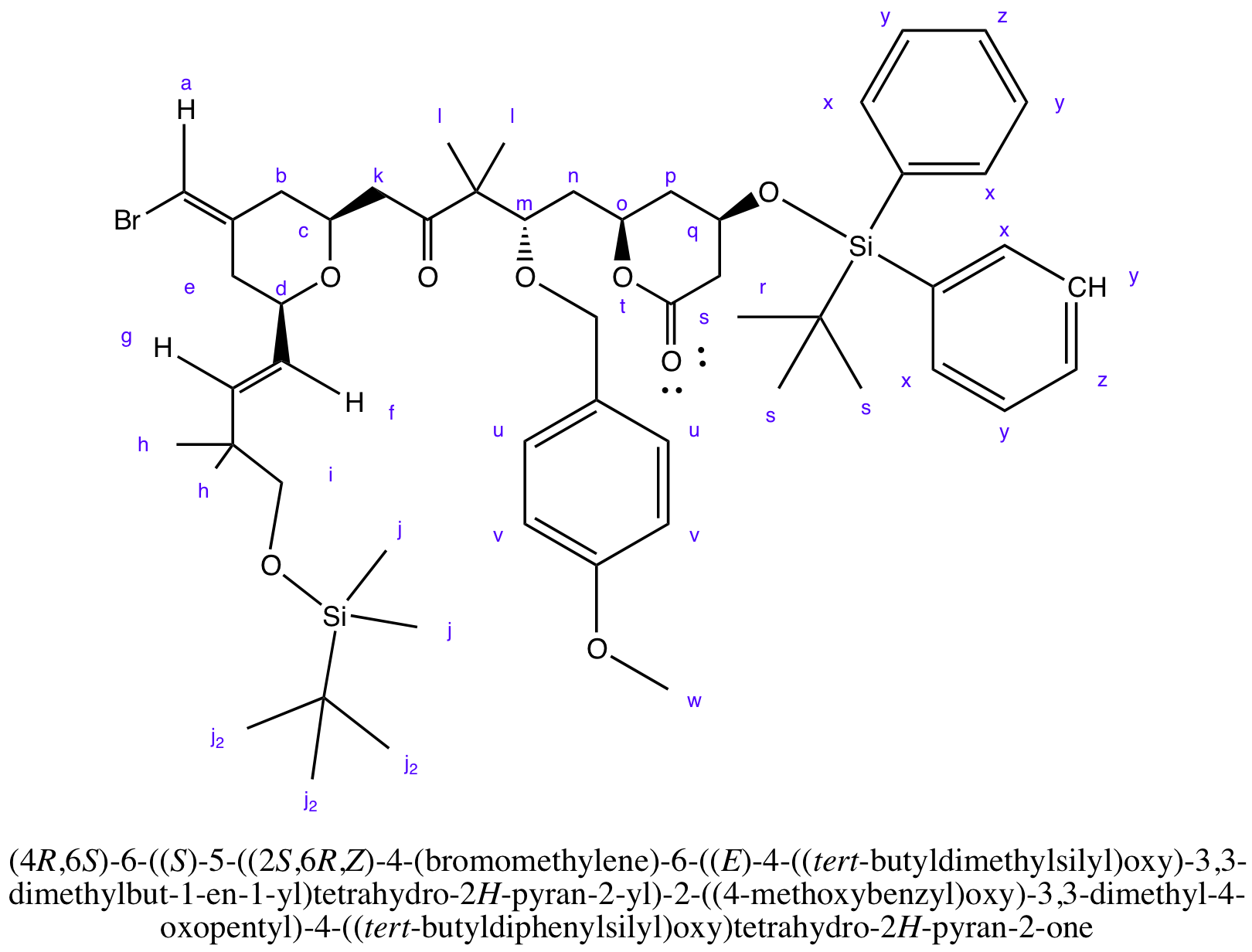
Compound 12
Compound 12 is a starting material, a lactone.
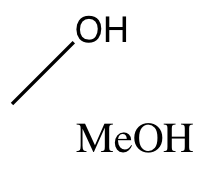
Dry MeOH is a weak, anhydrous acid.
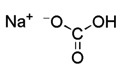
NaHCO3
Used to wash and remove any acidic impurities.
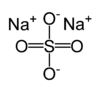
Na2SO4
Anhydrous sodium sulfate is used as a drying agent in the reaction.

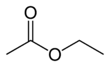
Ethyl Acetate
Ethyl acetate is an organic compound that is used as a solvent for our reaction for collection of organic compounds.
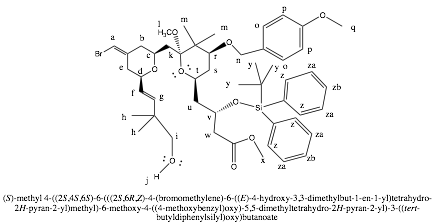
Compound 13
Compound 13 is our product.
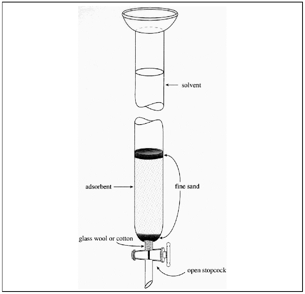
Silica Gel Flash Column Chromatography:
Column chromatography is used with silica gel to purify chemical compounds from another mixture. The process uses a glass tube with a filter and a wool plug at the bottom. A slurry of silica gel and distilled water is created while the silica is allowed to settle to the bottom of the column. A mixture passes through the silica gel and is collected in fractions and which are later analyzed. More polar products will pass through slower then less polar products due to bonding with the silica in the gel via hydrogen bonding.