Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Self Test: Finding WA
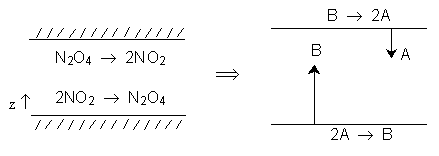
\( K_{\text{eqv}} = \frac{y_{N_2O_4}/2}{y_{NO_2}^2} \quad , \quad y_{N_2O_4} = \frac{\sqrt{1 + 4K} - 1}{2K} \)
Write the constitutive equation for NO2 solely as a function of yA, DAB, and WA.
WA = ?
\( A = NO_2 \quad B = N_2O_4 \)
\( 2A \longrightarrow B \)
\( W_A = -c D_{AB} \frac{dy_A}{dz} + y_A (N_A + N_B) \)
\( N_B = -\frac{1}{2} N_A \quad \left( W_A = -2 W_B \right) \)
\( W_A = \frac{-c D_{AB}}{1 - \frac{y_A}{2}} \frac{dy_A}{dz} \)
\( W_A = 2 c D_{AB} \frac{d}{dz} \ln \left( 1 - \frac{y_A}{2} \right) \)