Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Example: Measurement of Liquid Phase Diffusivities
A falling liquid jet is sometimes used to measure liquid-phase diffusivities of soluble gases. A jet of the pure liquid passes from a nozzle, designed to give a flat velocity profile, thence through a diffusion chamber containing the pure gas, and finally to a mixing vessel whose effluent is analyzed. For short jets it is usually a very good approximation to ignore curvature in the jet surfaces treating the process as diffusion into a fl at slab, since the concentration "boundary layer" is thin.
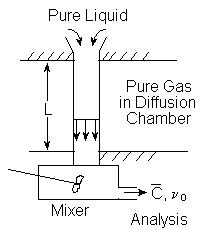
a. Assuming a perfectly flat velocity profile, constant jet diameter and negligible longitudinal diffusion, use the well-known relation for unsteady diffusion into a planar slab to derive a relation between
\( \overline{C} \) = mixed outlet concentration at steady-state
C* = equilibrium concentration of gas in liquid at chamber pressure and temperature (constants)
no volumetric flow rate of jet
L jet length
D diffusivity of gas in liquid (D º DAB)
b. In an experimental measurement of the diffusivity of CO2 in water, the mixed outlet concentration of CO2 is found to be
\( \overline{C} = 0.287 \times 10^{-6} \ \text{gm moles/cm}^3 \ \text{at } 25^\circ \text{C} \)
for a chamber pressure of 1 atmospheric CO2, and a jet length of 3 cm., and a flow rate of 12.0 cm3 /sec of water. What is the corresponding value of the diffusivity of CO2 in water?
c. Make an estimate of the maximum thickness of the CO2 boundary layer, i.e., penetration depth. Is the assumption negligible surface curvature justified for a 1 mm diameter jet?
\( P_{CO_2} = H X_{CO_2}^* = 1640 \, X_{CO_2}^* \)
Liquid Jet
For small penetration depths, the cross-sectional area for radial diffusion is equal to the surface area of the jet
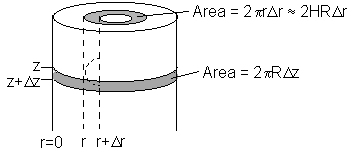
\( 2\pi R \Delta r W_{Az}\big|_z + 2\pi R \Delta z W_{Ar}\big|_r - 2\pi R \Delta r W_{Az}\big|_{z + \Delta z} - 2\pi R \Delta z W_{Ar}\big|_{r + \Delta r} = 0 \)
¸ by 2pRDrDz and take the limit as Dz and Dr go to zero
\( -\frac{\partial W_{Ar}}{\partial r} - \frac{\partial W_A}{\partial z} = 0(1) \)
\( W_{Ar} = -D_{AB} \frac{\partial C_A}{\partial r} \)
\( W_{Az} = -D_{AB} \frac{\partial C_A}{\partial z} + y_A (W_{Az} + W_{Bz}) \)
\( W_{Ar} = -D_{AB} \frac{\partial C_A}{\partial r} + y_A \left( W_A + W_{B_A} \right) \quad \text{(small concentration of A)} \)
In the axial (i.e., z) direction, the convective molar flux, \( y_A \left( W_{Az} + W_{Bz} \right) \) will be much greater than the molar flux by molecular diffusion, JA. One therefore can neglect JAz with respect to \( y_A \left( W_{Az} + W_{Bz} \right) \).
\( W_{Az} \approx y_A (W_{Az} + W_{BC}) = y_A C V_z^* = C_A V = C_A U \quad (3) \)
Substitution of Equations (3) and (2) into Equation (1) yields the following partial differential equation:
\( \frac{\partial C_A}{\partial z} = \frac{D_{AB}}{V} \frac{\partial^2 C_A}{\partial r^2} \quad (4) \)
with the boundary conditions:
\( C_A = C_{A0} \quad r = 0 \quad Z > 0 \)
\( C_A = 0 \quad r > 0 \quad Z = 0 \)
\( C_A = 0 \quad r \to \infty \quad Z \geq 0 \)
\( \text{Let } h = \frac{r}{z^n} \)
\( \frac{D_{AB}}{V} \frac{\partial^2 C_A}{\partial \eta^2} \cdot \frac{1}{z^{2n}} = \frac{-n}{z} \eta \frac{\partial C_A}{\partial \eta} \)
z can be eliminated from this equation by setting n = 1/2
\( \frac{D_{AB}}{V} \frac{d^2 C_A}{d\eta^2} + \frac{\eta}{2} \frac{d C_A}{d\eta} = 0 \)
with the boundary conditions:
\( C_A = 0 \quad \text{as } \eta \to \infty \)
\( C_A = C_{A0} \quad \text{as } \eta \to 0 \)
\( \text{Let } P = \frac{dC_A}{d\eta} \quad \text{and } D_{AB} = D \)
\( \frac{dP}{d\eta} = -\frac{\eta}{2D/V} P \)
\( P = \frac{dC_A}{d\eta} = A \exp\left[ \frac{-\eta^2 V}{4 D_{AB}} \right] \)
\( C_A - C_{A0} = A \int_0^{\eta} \exp\left[ \frac{-\eta^2 V}{4 D_{AB}} \right] d\eta \)
\( \text{As } \eta \to \infty, \quad C_A \to 0 \)
\( A = -\frac{C_{A0}}{\int_0^{\infty} \exp\left( \frac{-\eta^2 V}{4D} \right) d\eta} \)
\( \frac{C_A - C_{A0}}{C_{A0}} = \frac{\int_0^{\eta} \exp\left( \frac{-\eta^2 V}{4D} \right) d\eta} {\int_0^{\infty} \exp\left( \frac{-\eta^2 V}{4D} \right) d\eta} \)
\( \text{Let } y = \frac{\eta}{\sqrt{4D/V}} \)
\( \frac{C_{A0} - C_A}{C_{A0}} = \frac{ \int_0^{\eta} \exp\left[ -y^2 \right] dy }{ \int_0^{\infty} \exp\left[ -y^2 \right] dy } = \frac{2}{\sqrt{\pi}} \int_0^{\frac{\eta}{\sqrt{4D/V}}} \exp\left[ -y^2 \right] dy = \operatorname{erf} \left( \frac{\eta}{\sqrt{4D/V}} \right) \)
\( \operatorname{erf}(X) = \frac{2}{\sqrt{\pi}} \int_0^X e^{-y^2} \, dy \)
\( \frac{C_A}{C_{A0}} = 1 - \operatorname{erf} \left( \frac{r}{\sqrt{4Dz/V}} \right) \)
\( \frac{C_A}{C_{A0}} = \operatorname{erfc} \left( \frac{r}{\sqrt{4Dz/V}} \right) \)
Total rate of mass transfer = NAr
\( N_{Ar} = 2 \pi R \int_0^L W_{Ar}\big|_{r=0} \, dz \)
\( W_{Ar}\big|_{r=0} = -D_{AB} \left. \frac{\partial C_A}{\partial r} \right|_{r=0} = C_{A0} \sqrt{ \frac{D_{AB} V z}{\pi z} } \exp\left[ \frac{-r^2 V}{4 D z} \right] \Big|_{r=0} \)
\( W_{Ar}\big|_{r=0} = \sqrt{ \frac{D_{AB} V}{\pi z} } \, C_{A0} \)
\( N_{Ar} = 2 \pi R \int_0^L \sqrt{ \frac{D_{AB} V}{\pi z} } \, dz = 4 R \sqrt{ D_{AB} V \pi L } \, C_{A0} \)
\( N_{Ar} = \overline{C}_A \, v_0 \)
\( \frac{ \overline{C} }{ C_{A0} } = 4 \sqrt{ \frac{D_{AB} L}{v_0} } \)
\( C_{A0} = \frac{P}{H} \, C_t = 3.35 \times 10^{-5} \, \text{gmole/cc} \)
P = pressure of CO2 in the gas
H = Henry’s law constant
\( D_{AB} = \left( \frac{ \overline{C} }{ 4 C_{A0} } \right)^2 \frac{v_0}{L} \)
\( D_{AB} = \left( \frac{0.287 \times 10^{-6}}{4 \times 3.35 \times 10^{-5}} \right)^2 \cdot \frac{12}{3} \)
\( D_{AB} = 1.84 \times 10^{-5} \ \text{cm}^2/\text{sec} \)
\( \frac{C_A}{C_{A0}} = \operatorname{erfc} \left( \frac{r}{\sqrt{4 D z / V}} \right) \)
when CA = 0.01 CA0
\( 0.01 = \operatorname{erfc} \left( \frac{r}{\sqrt{4 D z / V}} \right) = \operatorname{erfc}(1.81) \)
r = d = thickness of the penetration zone
\( \delta = 1.81 \sqrt{ \frac{4 D_{AB} z}{V} } = 1.81 \sqrt{ \frac{4 D_{AB} L}{V} } = 1.81 R \sqrt{ \frac{4 D_{AB} \pi}{\pi R^2 V} } \)
\( \frac{\delta}{R} = 1.81 \sqrt{ \frac{4 D_{AB} \pi L}{Q} } = 6.7 \times 10^{-3} \)
d = 0.67 ´ 10–2mm or 1.3 percent of the radius; therefore, assumption of short penetration depths is justified.
Rather than using one experimental point to determine the diffusivity, one may vary the jet length to obtain a series experimental points. The total amount of CO2 absorbed is
\( \overline{C} = C_{A0} \cdot 4 \sqrt{ \frac{D_{AB} L}{v_0} } \)
\( W = 4 C_{A0} \sqrt{ D_{AB} L v_0 } \)
A plot of NAr vs.\( \sqrt{L} \) should be a straight line of slope
\( S = 4 C_{A0} \sqrt{v_0} \sqrt{D_{AB}} \)
\( D_{AB} = \frac{S^2}{16 \, C_{A0}^2 \, v_0} \)
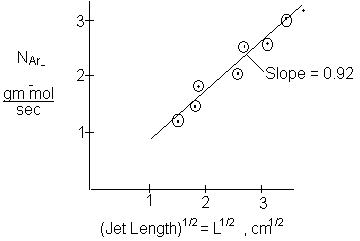
From the data of W. J. Thomas in J. Applied Chem.,17, p. 350 (1967)
\( D_{AB} \text{ at } 25^\circ \text{C} = 1.95 \times 10^{-5} \ \text{cm}^2/\text{sec} \)