Chapter 14: External Diffusion Effects on Heteregeneous Reactions
Equal Molar Counter Diffusion (EMCD)
(WA= -WB)or
Dilute Solutions (BA=0)
\( \text{Mole Balance} \): \( \frac{dW_Az}{dz} = 0 \)
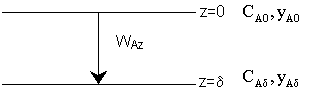
\( W_A = K_1 = -D_{AB} \frac{dC_A}{dz} \)
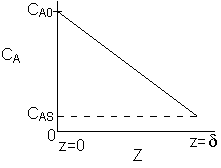
\( C_A = -\frac{K_1 z}{D_{AB}} + K_2 \)
at \( z = 0, \quad C_A = C_{A0} \)
\( K_2 = C_{A0} \)
at \( z = \delta, \quad C_A = C_{AS} \)
\( C_{AS} = \frac{K_1 \delta}{D_{AB}} + C_{A0} \)
\( K_1 = (C_{AS} - C_{A0}) \frac{D_{AB}}{\delta} \)
\( C_A = (C_{AS} - C_{A0}) \frac{z}{\delta} + C_{A0} \)
\( \frac{C_{A0} - C_A}{C_{A0} - C_{AS}} = \frac{z}{\delta} \)
\( \frac{y_{A0} - y_A}{y_{A0} - y_{AS}} = \frac{z}{\delta} \)
\( W_{Az} = -D_{AB} \frac{dC_A}{dz} = -D_{AB} [C_{AS} - C_{A0}] \frac{1}{\delta} \)