| Home | Experimental | References | NMR | Mechanism | About Us |
Reference found in article: Wadsworth, W. S.; Emmons, W. D. J. Am. Chem. Soc. 1961, 83, 1733-1738.
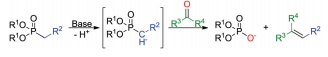
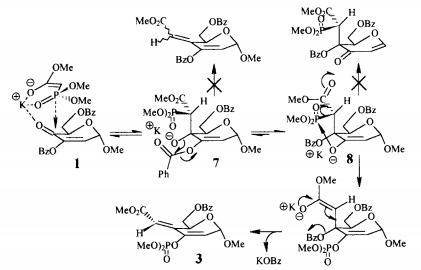
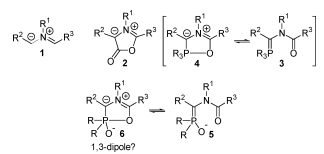
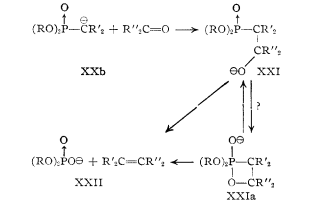
A key component of the transformation of molecule 3 to molecule 4 is the Horner-Wadsworth-Emmons reaction. This paper, written by Wadsworth and Emmons, details this mechanism in depth, in collaboration with ideas from a previous paper written by Emmons. In the first step of the reaction, a phosphonate anion adds to an aldehyde or ketone, forming an alkoxide intermediate. This intermediate is in equilibrium with a four-membered ring. The molecule then undergoes fragmentation yielding a phosphate anion and a newly formed double bond in the product. The paper discusses the benefits of using a phosphonate anion, because it reacts without reverse addition and can be used effectively with all ketones, even those that are sterically hindered. The phosphonate anion has three contributing resonance structures, giving the P=O bond some single bond character, which aid in its ability to react under mild conditions. The first step in the mechanism, where an aldehyde or ketone is added to the carbonyl group, yields an intermediate that is in equilibrium with a four-membered ring. These equilibrating structures then undergo fragmentation to yield the product and phosphate anion. Phosphonate anions can be used in conjunction with the Horner-Wadsworth-Emmons mechanism to yield a variety of different reaction products. For instance, they can react with alkyl halides to give α-substituted phosphonates, which will react with a hydride reagent and an aldehyde or ketone to give olefins, which is a type of synthetic fiber. Additionally, halogenation of a phosphonate anion with subsequent addition of an aldehyde or ketone will produce vinyl halides.