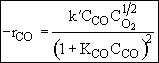![]()
The following data were obtained for the oxidation of CO over a catalyst. All rates are initial rates
|
|
|
|
|
.020 |
0.01 |
1 |
|
.035 |
0.01 |
3 |
|
.049 |
.01 |
6 |
|
.060 |
.01 |
9 |
|
.196 |
.1 |
1 |
|
.384 |
.2 |
1 |
|
.902 |
.5 |
1 |
|
1.653 |
1 |
1 |
|
4.44 |
5 |
1 |
|
5.00 |
10 |
1 |
|
4.44 |
20 |
1 |
|
2.77 |
50 |
1 |
The initial rate was found to be independent of CO2.
a) Suggest a rate law consistent with the data
Hint 1 Sketch![]() versus CO2.
versus CO2.
Hint 2 Sketch![]() versus CCO
at low concentration.
versus CCO
at low concentration.
Hint 3 Sketch![]() versus CCO
at high concentration.
versus CCO
at high concentration.
Full Solution What is the rate law?
b) Suggest mechanisms consistent with the rate law
Hint 1 What do the trends suggest?
Hint 2 What is the mechanism?
Use only the data points for which the concentration of CO is the same.
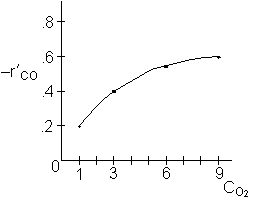
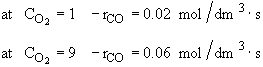
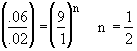
![]()
For constant CO2
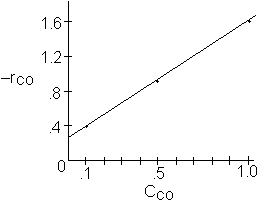
We see at low concentration the
rate is linear in CO and![]()
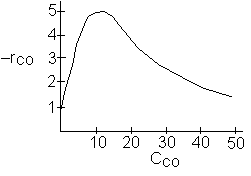
At high concentrations the rate decreases with increasing concentration
![]()
Combining
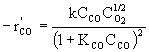
For fixed concentration of O2 and low concentration of CO and
![]()
![]()
![]()
High concentration
![]()
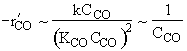
![]()
Consequently the rate law
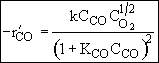
Satisfies the data
From the denominator of the rate law, we see that CO is adsorbed and that O2 is either weakly adsorbed (KO2PO21/2 << 1) or not adsorbed at all.
The 1/2 order with respect to oxygen suggests dissociative adsorption.
Because the initial rate is independent of CO2, it is either not adsorbed on the surface or weakly adsorbed.
The following mechanism is consistent with the rate law
Molecular
Adsorption ![]()
![]()
Dissociative
Adsorption ![]()
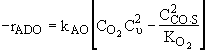
Surface
Reaction ![]()
![]()
The following mechanism is consistent with the rate law
Molecular
Adsorption ![]()
![]()
Dissociative
Adsorption ![]()
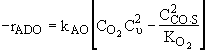
Surface
Reaction ![]()
![]()
Assume surface reaction limits
![]()
1) ![]()
![]()
2) 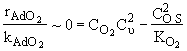
![]()
3) ![]()
4) ![]()
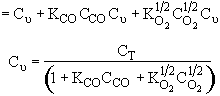
5) 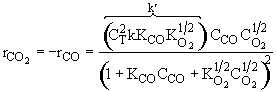
We see that oxygen is weakly
adsorbed (very small![]() ) such that
) such that![]()