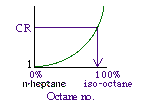
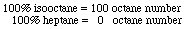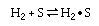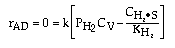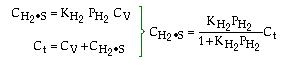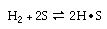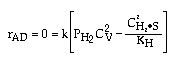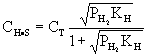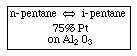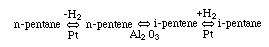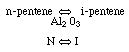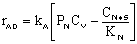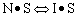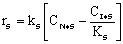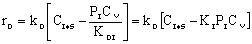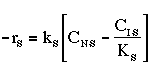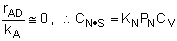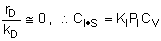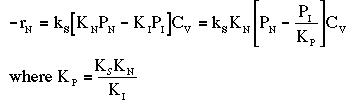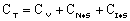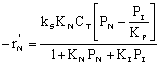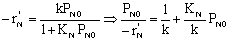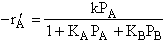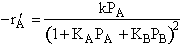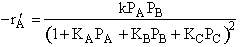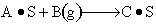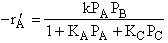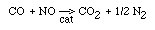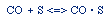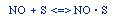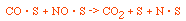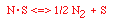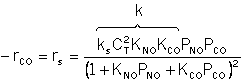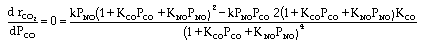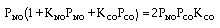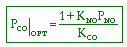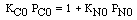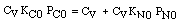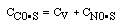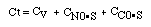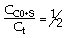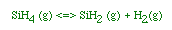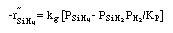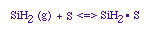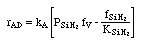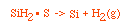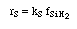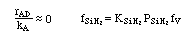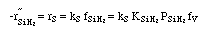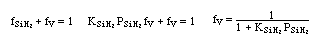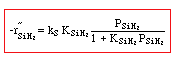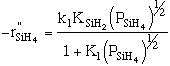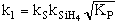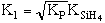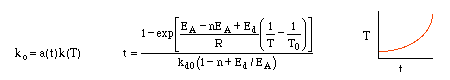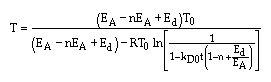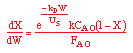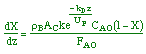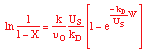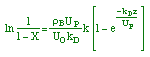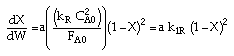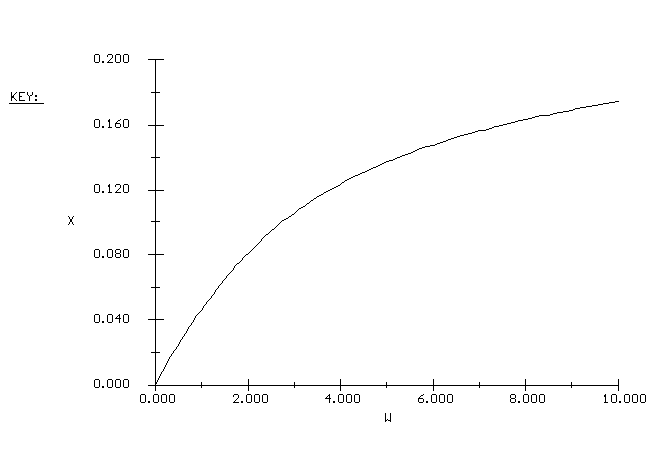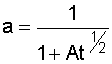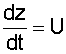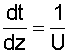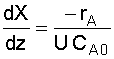|
|
|
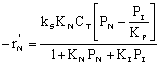
|
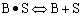
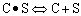
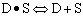
where KP is the thermodynamic equilibrium constant for the reactor. |
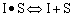
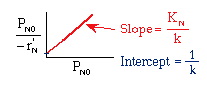
Linearizing the Initial Rate: |
|
 on setting rad/ka=0 on setting rad/ka=0
| Single site
|
| A)
|

|
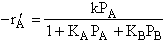
|
 |
| Dual Site
|
| B)
|

|
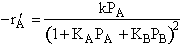
|

|
| C)
|
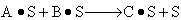
|
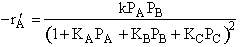
|

|
| Eley-Rideal
|
| D)
|
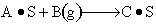
|
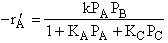
|

|
|
|
|
|
|

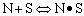
Finding the rate law and mechanism for
|
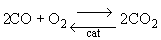 |

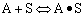
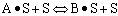
Finding the rate law and mechanism for A+B<=>C+D
|
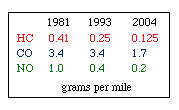
Regulations for Automotive Exhaust Emissions
|
top
|
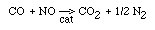
|
|
| Principle Reactions:
|
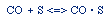
|
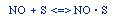
|
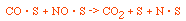
|
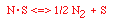
|
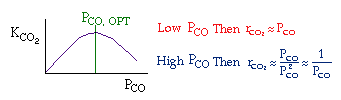
| Surface reaction limiting:
|
|
|
|
|
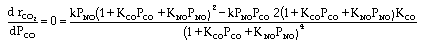
|
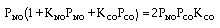
|
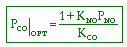
|

CO + NO; -r 'CO = f(P CO , P NO , P N2 , P CO2)
Example
| Let's see what fraction of sites are covered by CO at the
optimum:
|
|
|
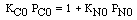
|
| Multiplying by CV:
|
|
|
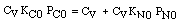
|
| (A)
|
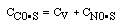
|
| (B)
|
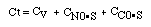
|
|
|
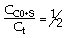
|

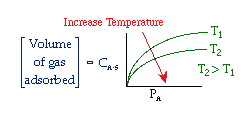
Calculating Fractional Coverage

Dimethyl Ether Examples

Example Exam Questions
|
Chemical Vapor Deposition, CVD (Chapter 10) |
top |
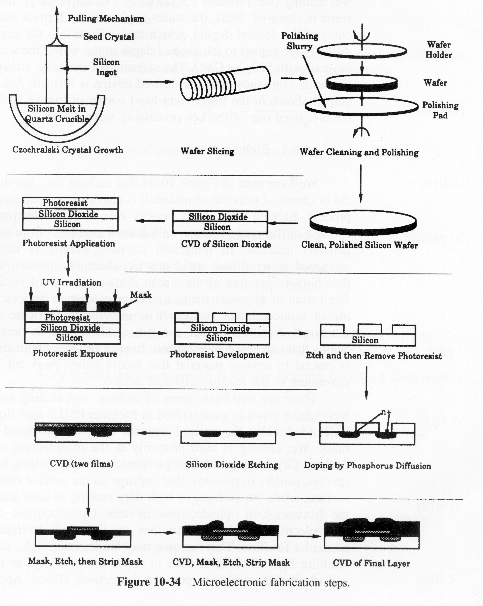
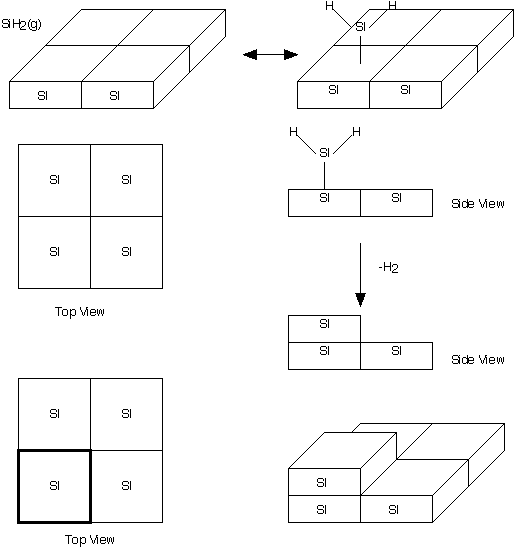
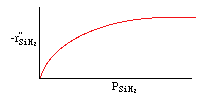
Manufacturing of a Silicon Layer
We see that a number of the key steps in the microelectronic
fabrication involve CVD, we shall consider the CVD of silicon.
| |
|
|
| For the homogeneous reaction: |
|
| |
then |
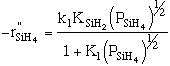  |
| |
| |
where: |
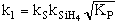
|
| |
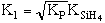
|
|
Types of Catalyst Deactivation
|
top
|
| Separable Kinetics: |
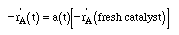 |
|
Types of Decay
| 1.) Sintering |
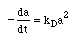 |
|
| 2.) Coking |
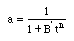 |
3.) Poisoning
|
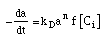 |
| 4.) Slow Decay |
Temperature-Time Trajectories |
| 5.) Moderate Decay |
Moving Bed |
|
| 6.) Rapid Decay |
STTR |
|
Temperature-Time Trajectories
|
top
|
The catalyst decay rate is a function of temperature, so you can vary the
temperature with time to keep the rate of decay as constant as possible.

Decaying Catalyst in a Batch Reactor
|
Moving Bed Reactors & Straight Through Transport Reactors
|
top
|
Catalyst Decay Example
The gas-phase, irreversible reaction
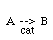 is elementary
with first order decay. The reaction is carried out at constant temperature and
pressure. is elementary
with first order decay. The reaction is carried out at constant temperature and
pressure.
|
Batch Reactor |
Moving Bed Reactor |
Straight Through
Transport Reactor |
| Mole Balance: |
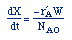 |
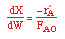 |
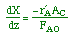 |
| Rate Law: |
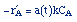 |
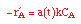 |
 |
| Decay Law: |
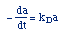 |
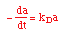 |
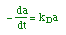 |
|
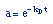 |
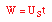 |
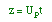 |
|
|
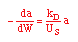 |
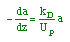 |
|
|
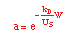 |
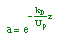 |
| Stoichiometry: |
gas phase, but
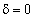 , T = T0, and P = P0 , T = T0, and P = P0 |
|
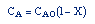 |
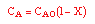 |
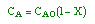 |
| Combine: |
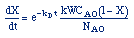 |
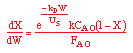 |
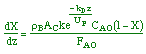 |
|
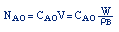 |
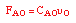 |
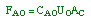 |
|
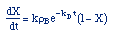 |
|
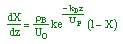 |
|
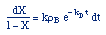 |
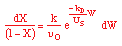 |
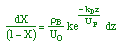 |
|
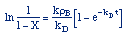 |
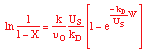 |
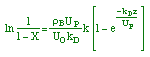 |
Another Catalyst Decay Example
The second-order, irreversible reaction
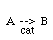 is carried out
in a moving bed reactor. The catalyst loading rate is 1 kg/s to a reactor
containg 10 kg of catalyst. The rate of decay is second order in activity and
first order in concentration for the product, B, which poisons the catalyst.
Plot the conversion and activity as a function of catalyst weight down the
reactor. is carried out
in a moving bed reactor. The catalyst loading rate is 1 kg/s to a reactor
containg 10 kg of catalyst. The rate of decay is second order in activity and
first order in concentration for the product, B, which poisons the catalyst.
Plot the conversion and activity as a function of catalyst weight down the
reactor.
Additional information:
Solution:
| Polymath |
| Mole Balance: |
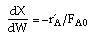 |
| Rate Law: |
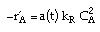 |
| Decay Law: |
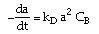 |
|
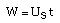 |
|
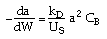 |
|
| Stoichiometry: |
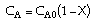 |
|
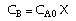 |
| Combine: |
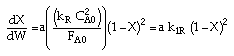 |
|
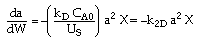 |
|
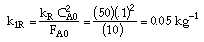 |
|
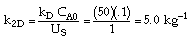 |
| Conversion vs. Catalyst Weight |
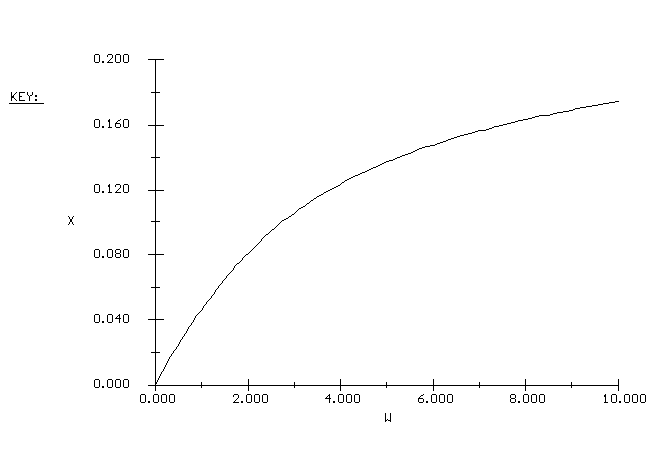 |
| Catalyst Activity vs. Catalyst Weight |
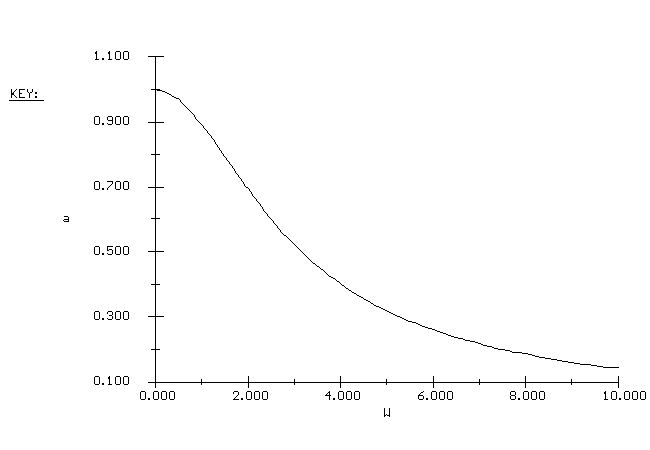 |

Moving Bed Reactor
Example 10-7: Strictly Speaking

Catalyst Decay in a Packed Bed

Object Assessment of Chapter 10
*
All chapter references are for the 4th Edition of the text Elements of Chemical Reaction Engineering
.
top
|