| Additional information: | Sample Data | ||||
|---|---|---|---|---|---|
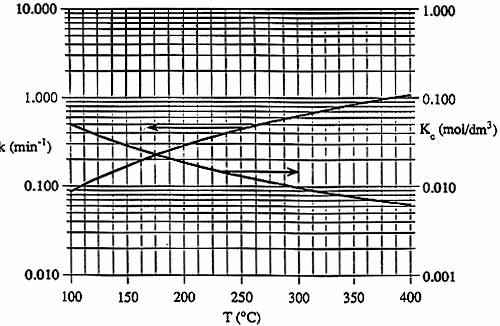
| Graphs of k(T) and KC are shown below. | T | k | KC | Xe | |
| CpA = 5.0 cal/(mol - šC) | šC | min-1 | mol/dm3 | -- | |
| CpB = 2.5 cal/(mol - šC) | 100 | 0.086 | 0.05 | 0.75 | |
| CpC = 5.0 cal/(mol - šC) | 200 | 0.27 | 0.018 | ||
| 300 | 0.62 | 0.0096 | |||
| 400 | 1.05 | 0.006 | 0.36 | ||
(25 pts)
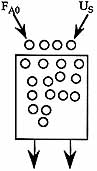
3) The elementary reaction
is carried out in a moving bed reactor in which the catalyst decays by sintering. What catalyst weight is necessary to achieve 50% conversion for a catalyst loading rate of 5 kg/min? Pure A is fed to the reactor at a concentration of 0.2 mol/dm3 and a molar flow rate of 5.0 mol/min.
| Additional Information | |
|---|---|
| Specific Reaction Rate | k=1.0 dm6 /(mol-kg cat-min) |
| Catalyst decay constant | kd=0.04 min-1 |
(15 pts)
3) The elementary reaction
is irreversible and carried out adiabatically in a CSTR. The R(T) and G(T) (kcal/mol) are shown in the attached figure.
- (a)What is the heat of reaction for this reaction?
- (b)What is the heat capacity of species A?
- (c)What is the entering temperature at which ignition will occur?
- (b)What is the heat capacity of species A?
Back to the exam list