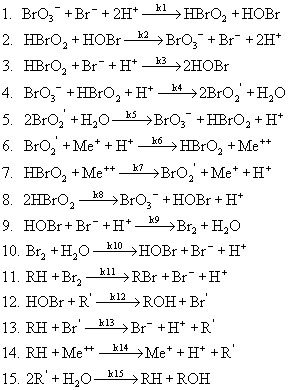
Now, going back to the actual BZ reaction scheme itself, as mentioned earlier there are over 40 reactions to consider. Many of these reactions are minor, and so we are able to reduce the scheme to just 15 reactions, which are shown below.
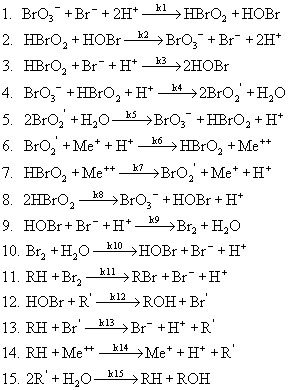
In the above scheme, BrO2', R', and Br' are free radical intermediates. RH is malonic acid and Me represents a metal ion. Notice that reactions #1 and 2, #4 and 5, #6 and 7, and #9 and 10 are essentially the same reaction, with one case being the reverse of the other. Because of this, in writing the rates of reaction we can reduce the number from 15 to 11. These 11 rates are shown below, with respect to a single mole of reactant.
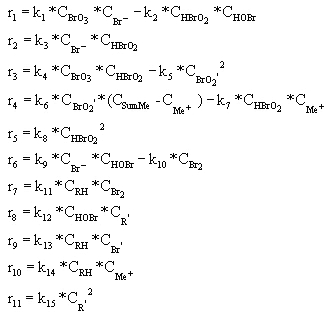
CBrO3 represents the stationary concentration of BrO3-, CRH represents the stationary concentration of RH, and CSumMe is the sum of the Me+ and Me++ concentrations. Putting the BZ scheme into Polymath with the appropriate rate constants, we get the following program.
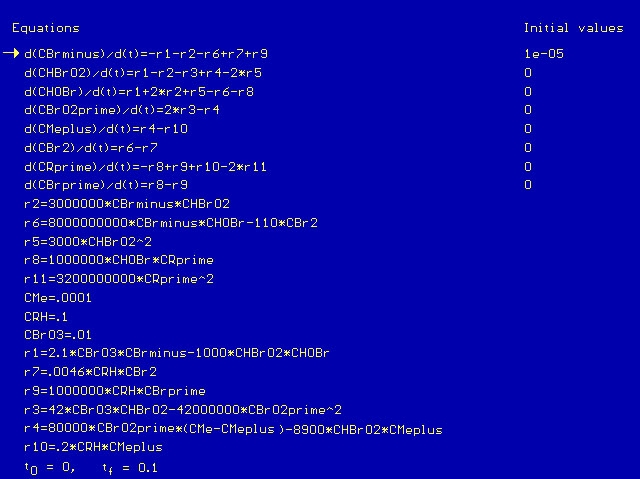
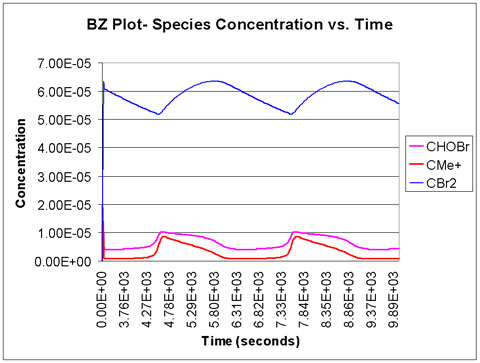
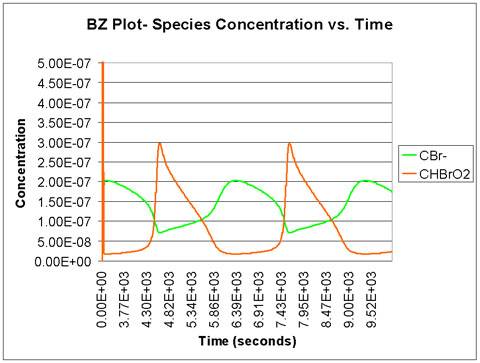
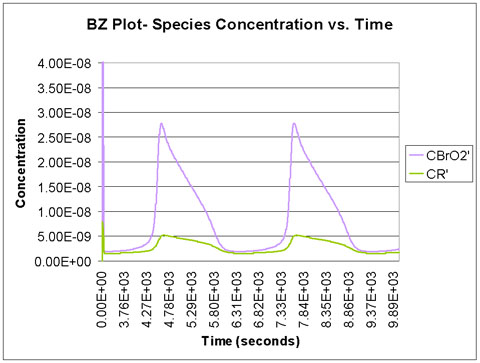
We run the program with a time scale of about 3 hours. The species concentrations as a function of time are shown below. We see that all eight major species do in fact oscillate with time.