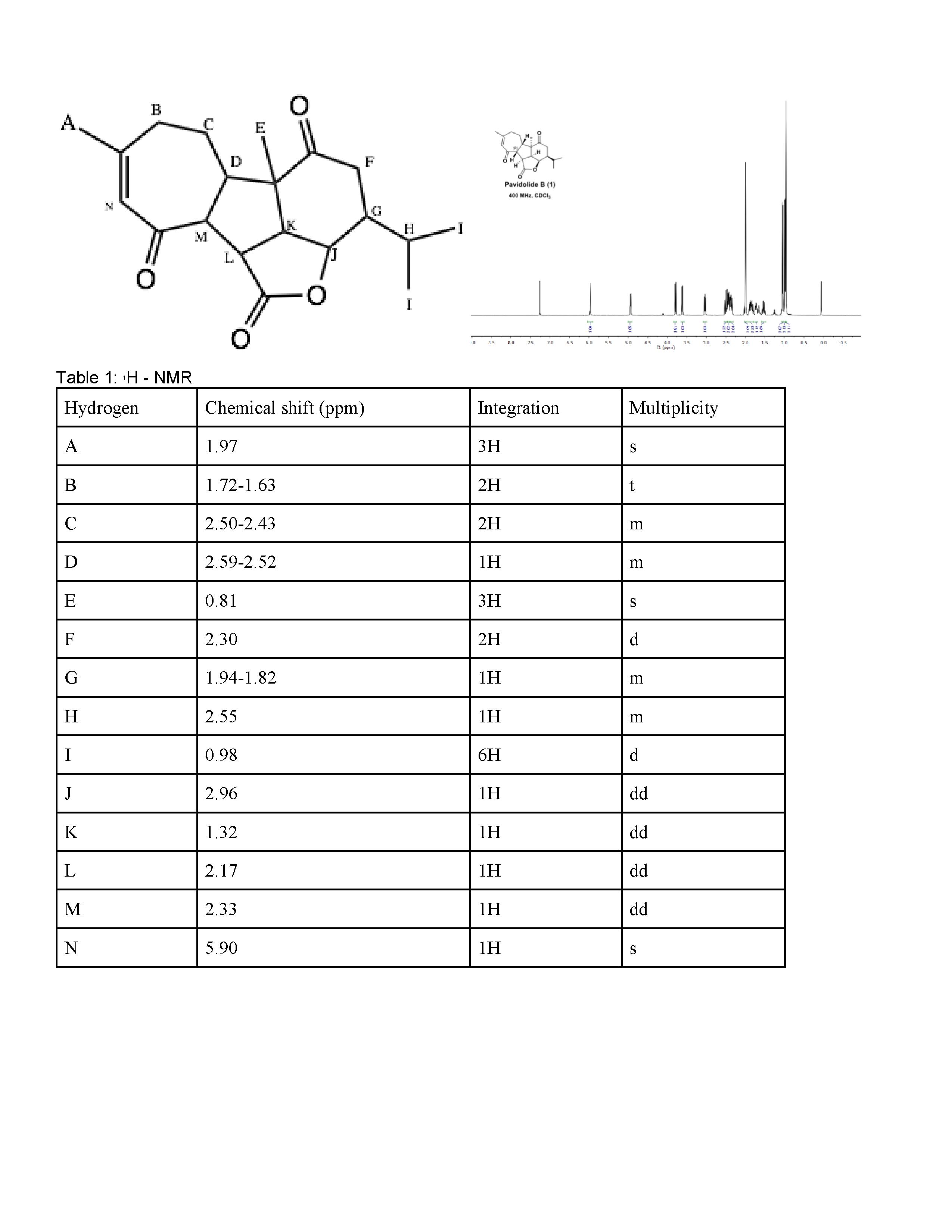
There is only 6H group, which must correspond to I because that is the only 6H group. Next, there are three 3H groups, all of which are doublets. A must be the 1.60 ppm because it is farthest away from the electron donating double bonded oxygen. However, it is very difficult to tell the difference between E and P because they are both methyl groups close to an electron donating group which would result in a very low ppm. The hydrogens at R are diastereotopic but due to how similar they are, they are probably around the same ppm. Between F and N, it is nearly impossible to tell the difference between the two because they are both next to electron donating double bonded oxygen groups. C is on a double bond while D is closer to the electron withdrawing group. This means D should have a lower ppm than C, which is does at 2.65, and D should be around 5.0 ppm which it is, 5.33-5.28. J has the highest ppm at 3.14 because it is closest to the electron withdrawing group and is thus the most deshielded. K is the least deshielded because it is farthest away from the electron withdrawing group. However, M and L are nearly identical because they are both adjacent to electron withdrawing carbonyls. H should have the lowest ppm, at 1.85-1.78 because it is farthest from the electron withdrawing group and is thus the most shielded. H should have the next highest ppm at 3.67-3.56 because it is closer to the electron withdrawing group and is thus more deshielded. Next, Q and B must be similar because they are both on the ends of a double bond. Therefore, Q and B correspond to 5.79-5.72 ppm and 5.33-5.28 respectively because those are two values both close to 5.0 ppm. This means the last peak 4.8 must correspond to hydrogen O which makes sense because it is closer to the electron donating group and thus should have a lower ppm.
