Vanadium Project
Introduction
Vanadium has been found to play a number of roles in biological systems. It is present in certain vanadium dependent haloperoxidase and nitrogenase enzymes. Many sea squirts accumulate vanadium in very high concentration, although the reason is not known. The Amanita muscaria mushrooms also accumulate vanadium in the form of a coordination complex called amavadin, whose function is also unknown. Finally, a number of vanadium complexes have been shown to alleviate many of the symptoms of diabetes in both in vitro and in vivo (in rats and mice) studies. These complexes are being studied as potential alternatives to insulin therapy.
Research in the Pecoraro group has concentrated primarily on designing spectroscopic and functional models for vanadium dependent haloperoxidases (VHPOs). VHPOs catalyze the 2-electron oxidation of a halide (X- = Cl-, Br-, or I-) by peroxide through a Lewis acid-promoted mechanism (as opposed to redox cycling at the vanadium center) as summarized in Scheme 1. A reactive halogenating species (HOX, X2, or X3-) is produced which can react with electron-rich substrates to form halogenated organic compounds. Vanadium remains in the +5 oxidation state throughout the entire catalytic cycle. When the vanadium is reduced to the +4 oxidation state, the enzyme is totally inactive.
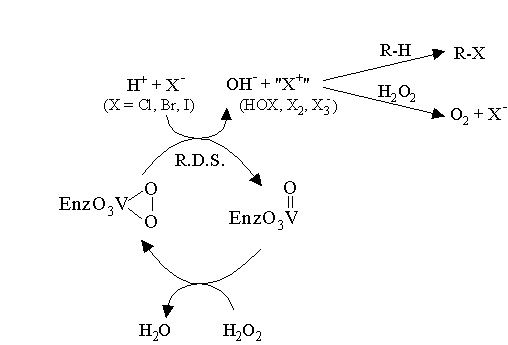
Scheme 1. Proposed mechanism for VHPOs: Vanadium activates hydrogen peroxide towards nucleophilic halide attack through a lewis acid mechanism.
The crystal structure of the native and peroxide bound form of VClPO from Curvularia inaequalis have been reported by Messerschmidt, Prade and Wever (Biol. Chem.,, 1997, 378, 309). The coordination environment for the active species is based on this structure and is presented in figure 1. The trigonal bipyramidal vanadium is coordinated to four non-protein oxygen donors (O2-, OH-, or OH2) and a histidine. Another nearby histidine is believed to act as an acid-base catalyst. The crystal structures of two VBrPOs have also been solved: the dodecameric VBrPO from Corallina officinalis (Isupov, M.N, et al., J. Mol. Biol., 2000, 299, 1035) and the dimeric enzyme from Ascophyllum nodosum (Weyand et al., J. Mol. Biol., 1999, 293, 595). While all three have vastly different gross structures, the active sites are all highly conserved with the VBrPOs having one more hitidine in the active site than the VClPO.
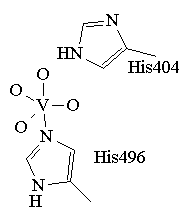
Figure 1. The trigonal bipyrimidal coordination environment proposed for the active form of VCIPO.
Project Goals
- Use EPR, ESEEM (with the Frasch and LoBrutto groups at Arizona State), and ENDOR (with the Britt group at UC-Davis) to explore spectroscopic models for the reduced form of vanadium haloperoxidase (VHPO) and to acquire a library of data for known compounds so vanadium(IV) can be more widely used as a spectroscopic probe in other biological systems.
- Improve on our functional models for VHPO, including [VO(O2)(Hheida)]- (Scheme 1), which is already the most efficient model known to date.
- Investigate the amavadin model complex [V(hida)2]2-, looking for an answer for amavadin's role in nature.
- Apply the knowledge gained from our research in vanadium chemistry to broader areas, such as vanadium's insulin mimetic activity.
Current Work
We have recently synthesized and characterized several peroxovanadium complexes which act as functional models for the vanadium haloperoxidases. Figure 2 shows the structure of [VO(O2)Hheida]-, one of these complexes. The distorted pentagonal bipyramidal structure and the side-on bound peroxo ligand are typical for complexes of this type. In acetonitrile solution these compounds rapidly and efficiently catalyze the two-electron oxidation of bromide and iodide upon the addition of acid, as can be observed either by monitoring the formation of trihalide or by monitoring the halogenation of organic substrates such as Phenol Red. Upon the addition of excess hydrogen peroxide in the absence of substrate, dioxygen is produced via the halide-assisted disproportionation of hydrogen peroxide.
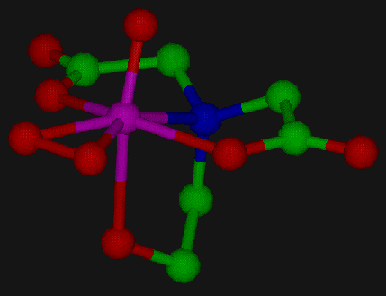
Figure 2. This figure represents the crystallographic structure of the molecule [VO(O2)Hheida], one of a series of catalytic models for the vanadium haloperoxidase enzyme.
Kinetic experiments are consistent with a mechanism which is first order in halide and vanadium complex, and kinetic and mechanistic experiments reveal that protonation of the complex is essential for the halide oxidation reaction to occur. Rate constants for halide oxidation and equilibrium constants for the protonation of the complexes have been obtained from the kinetic data. A proposed mechanism for the model compounds studied (and by extension the haloperoxidases) is illustrated in Scheme 2. This mechanism is consistent with the kinetic and structural information reported for the enzymes given above, and lends support to the proposal that an acid/base catalyst is essential to the enzymes' activity.
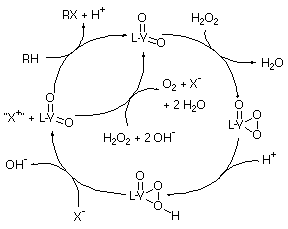
Scheme 2. The catalytic cycle proposed for VHPOs based on our work.
Current research on these systems focuses on probing the mechanism of formation of peroxovanadium complexes under these conditions, and on pinpointing the site of protonation which activates these complexes toward halide oxidation. We also seek to understand how structural changes in the complexes affect their reactivity.
On a related note, we have also begun to investigate the structural and spectroscopic properties of vanadium(IV) complexes with these ligands. Figure 3 illustrates the crystal structure of one of these complexes. We believe that a combination of crystallography, UV/visible spectroscopy, and continuous-wave and pulsed EPR spectroscopies is likely to provide important insights into the structures of the reduced, inactive forms of the vanadium-dependent haloperoxidases and other vanadyl-substituted proteins.
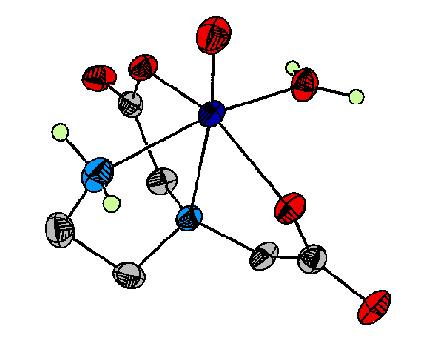
Figure 3. This molecule has been used as a spectroscopic model for vanadium (IV) binding to proteins such as the chloroplast coupling factor.
Modern computational methods have made it possible to predict the configuration and behavior of molecules and complexes enabling us the better design small molecule models. We are using an active site model which includes several small molecules which are representative of the side chains involved in the hydrogen bonding network of the active site. Upon minimization of the resting structure, we will simulate the sequential binding of substrates (H2O2, H+, halide) in various binding modes in order to elucidate their position and orientation during catalysis and hence which residues are involved in the reactions.
Additivity Relationship of Vanadyl Complexes in EPR
The additivity relationship for vanadyl complexes is a useful tool that correlates the hyperfine coupling constant (all) from EPR spectra to the types of ligands bound equatorially to vanadium. Unfortunately, use of this tool has been hampered by ambiguity in the additivity value for imidazole. To better determine the additivity relationship value for imidazole, we have synthesized several new vanadyl complexes (such as the one in Figure 4) with bound imidazole.
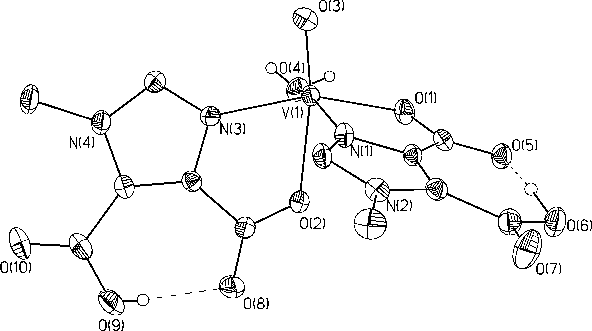
Figure 4. This is one of the molecules which has been used to determine the additivity relationship which correlates the hyperfine coupling constant (all) from EPR spectra to the types of ligands bound equatorially to vanadium.
Together with the four previously crystallographically characterized vanadyl-imidazole complexes, additivity values were determined for imidazole. These values fell predominately into two groups, which correlated to the orientation of the imidazole ring relative to the vanadyl unit. When the ring makes a small angle to the vanadyl unit it has a much smaller value (~40 x 10-4 cm-1) than when it is 90 degrees away (~46 x 10-4 cm-1), and the data may be fit to a sine curve (See Figure 5). There is also preliminary evidence that orientation affects the contribution of other imine-type donors as well.
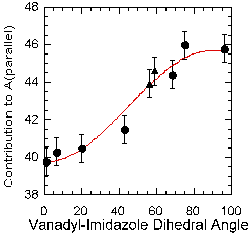
Figure 5. Sine curve fitting of the relationship between the vanadyl-imidizole dihedral angle and the contribution that imidizole makes to All
Recent Project Publications (1995-present)
- Dissertations:
- Brent Hamstra, 1997, "Vanadium-Aminocarboxylate Complexes: Models for the Roles of Vanadium in Biological Systems".
- Thomas S. Smith II, 2001 , "Exploring Reactive and Spectroscopic Models of the Vanadium Haloperoxidases"
- Joslyn Yudenfreund Kravitz , 2005 , "Computational Studies of the Vanadium Dependent Haloperoxidases and Vanadyl-Imidazyol Complexes"
- Undergraduate Thesis:
- Jason Walter Kieltyka, 2000, "Strategies for Modeling the Vanadium Haloperoxidase".
- Journals, Periodicals, and Books:
- Colpas, G.C.; Hamstra, B.J.; Kampf, J.W.; Pecoraro, V.L. "Functional Models for Vanadium Haloperoxidase: Reactivity and Mechanism of Halide Oxidation" J. Am. Chem. Soc., 1996, 118, 3469.
- Slebodnick, C.; Hamstra, B.J.; Pecoraro, V.L. "Modelling the Biological Chemistry of Vanadium: Structural and Reactivity Studies Elucidating Biological Function", Structure and Bonding, P. Sadler, Ed., 1997, 89, 57.
- Slebodnick, C.; Hamstra, B.J.; Pecoraro, V.L.; "Modeling the Biological Chemistry of Vanadium: Structural and Reactivity Studies Elucidating Biological Function", Metal Sites in Proteins and Models, 1997, 89, 51-108.
- LoBrutto, R.; Hamstra, B. J.; Colpas, G. J.; Pecoraro, V. L.; Frasch, W. D.; "ESEEM Spectroscopy Reveals and Distinguishes Equatorial and Axial Nitrogen Ligands Bound to VO2+"; J. Am. Chem. Soc., 1998, 120, 4410-4416.
- Hamstra, B. J.; Houseman, A. L. P.; Colpas, G. J.; Kampf, J.W.; LoBrutto, R.; Frasch, W. D.; Pecoraro, V. L.; "Structural and Solution Characterization of Mononuclear V(IV) Complexes that Help to Elucidate the Active Site Structure of the Reduced Vanadium Haloperoxidases", Inorg. Chem., 1997, 36, 4866.
- Hamstra, B. J.; Gillis, M.; Colpas, G. J.; Kampf, J. W.; Pecoraro, V. L.; "An Analysis of the Structural Reorganization Obsereved in V(IV) and V(V) Aminocarboxylate Complexes: Implications for the Reactivity of V(IV) and V(V) Complexes with Hydrogen Peroxide", Inorg. Chem.,
- Hamstra, B. J.; Pecoraro, V. L.; "Reactivity of Dioxovanadium(V) Complexes with Hydrogen Peroxide: Implications for Vanadium Haloperoxidases", Inorg. Chem., 1998, 37, 949-955.
- Grant, C.V.; Ball, J.A.; Hamstra, B.J.; Pecoraro, V.L.; "V51 Studies of Oxovanadium(IV) Complexes: Investigation of the Nuclear Quadrupole Interaction", Jour. Phys. Chem. B, 1998, 102, 8145-8150.
- Slebodnick, C.; Pecoraro, V.L.; "Solvent Effects on V51 Shifts: Characterization of Vanadate and peroxovanadate Complexes in Mixed Water/Acetonitrile Solvent", Inorg. Chim. Acta, 1998, 283, 37-43 (Special Issue Devoted to Osamu Yamauchi).
- Thomas S. Smith, II, Charles A. Root, Jeff W. Kampf, Paul G. Rasmussen, and Vincent L. Pecoraro, "Reevaluation of the Additivity Relationship for Vanadyl-Imidazole Complexes: Correlation of the EPR Hyperfine Constant with Ring Orientation", J. Am. Chem. Soc., 2000, 122, 767-75.
- Smith II, T.S.; LoBrutto, R.; Pecoraro, V.L. "Paramagnetic Spectroscopy of Vanadyl Complexes: Applications to Biological Systems," Coord. Chem. Reviews, 2002, 228, 1-18.
- Smith II, T.S.; Pecoraro, V.L. "Oxidation of Organic Sulfides By Vanadium Haloperoxidase Model Complexes," Inorg. Chem., 2002, 41, 6754-6760.
- Zampella, G; Kravitz, J.Y.; Webster,C. E.; Fantucci, P.; Hall, M. B.; Carlson, H. A.; Pecoraro, Vincent L.; Gioia, L. D.; "Quantum Mechanical Models of the Resting State of the Vanadium-Dependent Haloperoxidase" Inorg. Chem., 2004, 43, 4127-4136.
- Zampella, G.; Fantucci, P.; Pecoraro,V.L.;, and Gioia, L. D.;; "Reactivity of Peroxo Forms of the Vanadium Haloperoxidase Cofactor. A DFT Investigation" J. Am. Chem. Soc. 2005, 127, 953-960
- Kravitz, J.Y. and Pecoraro, V. L.; " Synthetic and computational modeling of the vanadium-dependent haloperoxidases", Pure Appl. Chem. 2005 , 77(9), 1595-1605.
- Zampella, G.; Fantucci, P.; Pecoraro,V.L.;, and Gioia, L. D.;; "Insight into the Catalytic Mechanism of Vanadium Haloperoxidases. DFT Investigation of Vanadium Cofactor Reactivity." Inorg. Chem., 2006, 45(18), 7133-7143.
- Schneider, C. J.; Zampella, G.; Greco, C.; Pecoraro, V. L.; Gioia, L. D.;; "Mechanistic analysis of nucleophilic substrates oxidation by functional models of vanadium-dependent haloperoxidases: a density functional theory study", Eur. J. Inorg. Chem., 2007, (4), 515-523.
- Schneider, C.J.; Pecoraro, V.L.; "Understanding the Mechanism of Vanadium Dependent Haloperoxidases and Related Biomimetic Catalysis," ACS Symposium Series, 2007, 974, Chapter 12, 148-162.
- Schneider, C.J.; Penner-Hahn, J.E.; Pecoraro, V.L.; "Elucidating the Protonation Site of Vanadium Peroxide Complexes and the Implications for Biomimetic Catalysis" J. Amer. Chem. Soc., 2008, 130, 2712-2713.
- Micera, G.; Pecoraro, V.L.; Garribba, E. “Assessing the Dependence of 51V Az Values on the Aromatic Ring Orientation of VIVO2+ Pyridine Complexes,” Inorg. Chem. 2009, 48, 5790-5796.