Exploiting and Controlling the Dynamics of Molecules
The cis - trans isomerization of polyenes is exploited in natural and artificial systems to achieve a desired outcome. Optical excitation results in a change in shape and can result in a change in chemical properties. Rhodopsins use the isomerization of retinal chromophores as photoreceptors in vision and to power proton pumps in bacteria. Molecular motors harness unidirectional photoisomerization to produce motion. Merocyanine based photoacids exploit trans to cis isomerization of a polyene backbone to produce a local change in pH.
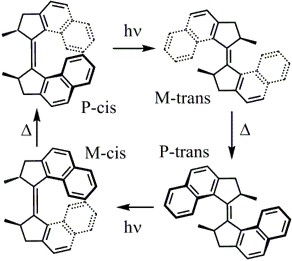
The first generation molecular motor shown to the left was studied using transient absorption spectroscopy. The transient difference spectrum for the P-trans to M-cis isomerization reaction is shown in the figure below. The M-cis molecule is formed within 13 ps. The P-cis to M-trans trans reaction is slower, with M-trans formed on a 70 ps time scale. Both photochemical steps are efficient with quantum yields exceeding 80%.
For the first generation motor above, optical manipulation using pulse sequences is capable of increasing the yield of the cis to trans isomerization, but only decreases the yield of the trans to cis isomerization. Future studies will explore the ability of shaped pulses or pulse sequences to modify the yields in second generation motors where the intrinsic quantum yield for the photochemical step is much lower (<20%).
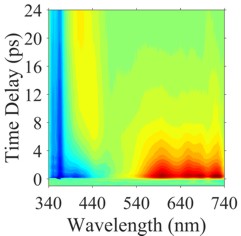
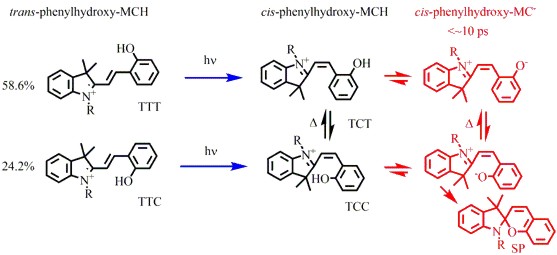
The metastable merocyanine photoacid shown below was studied using ultrafast transient absorption spectroscopy and state-of-the-art theoretical simulations. Photoisomerization from the trans to cis conformation results in prompt deprotonation within 10 ps. This is truly an ultrafast photoacid. Standard theoretical calculations do not predict this rapid deprotonation. More sophisticated QM/MM simulations are required to dissect the mechanism for the reaction.
Future work will exploit this ultrafast metastable photoacid and similar compounds to produce rapid changes in pH in a confined environment. This will permit the use of ultrafast time-resolved spectroscopies from the near IR to the X-ray region to investigate ground state acid-base reactions.
This research is supported by the National Science Foundation