State-of-the-art femtosecond to nanosecond time-resolved spectroscopic techniques are being used to study excited state dynamics and photochemistry in metal-centered cyclic tetrapyrroles. Current research is focused on investigating the excited state dynamics of cobalamins (vitamin B12 and analogs).
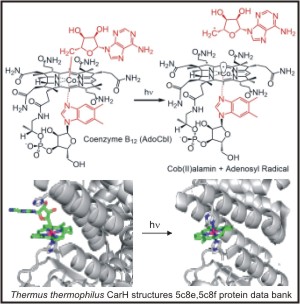
The key catalytic element in B12 is a unique carbon - cobalt bond. Reactivity in B12 enzymes involves homolysis (adenosylcobalamin dependent enzymes) or heterolysis (methylcobalamin dependent enzymes) of the active carbon-cobalt bond. Recently a class of B12 dependent photoreceptors have been identified. CarH uses B12 photolysis to regulate gene expression and carotenoid synthesis.
A photon can be used to trigger bond homolysis in both enzyme bound and free B12 compounds. Our work focuses on the influence of the axial ligand and environment on the excited state dynamics and photochemistry of cobalamins.
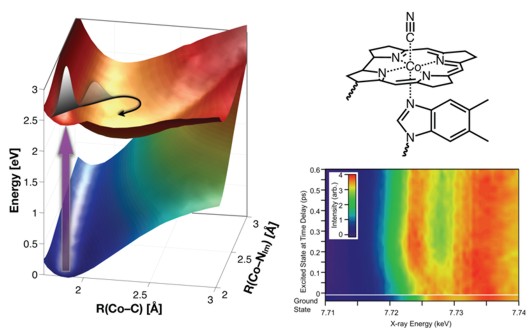
Ultrafast time-resolved X-ray spectroscopy provides the opportunity to make "molecular movies" by monitoring the coherent structural changes of a reactive molecule. The XANES measurement is particularly sensitive to the structure and dynamics of the ligands surrounding the reactive central cobalt atom. Polarization can be used to separate the axial and equatorial motions and define the sequence of molecular transformations in the excited electronic state. Our initial studies of cyanocobalamin are being expanded to include a much larger range of cobalamin compounds.
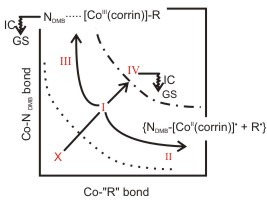
The photochemistry of cobalamins is governed by seams between at least three excited electronic states. The location of these seams depends on the bonding between the cobalt and the upper and lower axial ligands. The surface is shown schematically in the figure below with alternative reaction pathways indicated.
Femtosecond X-ray spectroscopy at the Co K-edge is used to monitor the structural evolution of the axial and equatorial ligands following optical excitation. The X-ray absorption near edge structure (XANES) reveals ballistic motion of the molecule on the excited state potential energy surface following excitation. Excitation of vitamin B12 (cyanocobalamin) results in a slight enlargement of the Co cavity within 15 fs followed by elongation of the axial bonds reaching a maximum at 190 fs. This is followed by contraction to the stable excited state structure within 500 fs.
This research is supported by the National Science Foundation. Use of the Linac Coherent Light Source (LCLS), SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515.