APPLICATIONS OF ION MOBILITY-MASS SPECTROMETRY TO DISEASE-ASSOCIATED MULTI-PROTEIN SYSTEMS
We focus much of our work on building models of unknown or partially unknown multi-protein systems involved in disease. Our general aim is to develop clear, testable models for multi-protein complexes that have refused characterization using the more-established tools of structural biology. It is our hope that the coarse-grain models we develop using the IM-MS approaches described elsewhere on this site will enable the future generation of higher-resolution structures of these complexes through the integration of IM-MS constraints with other structural data and modeling techniques. We have chosen to focus our initial model building efforts in two main areas: DNA Replication / Cancer and Amyloid Formation /Amyloid-related Diseases. Our DNA replication work is done in collaboration with groups at the University of Oxford [Robinson], California State University [Barsky], The Rockefeller University [O’Donnell], and the University of Wollongong [Dixon, Beck]. Our Amyloid work is currently performed in collaboration with groups at the University of Michigan (specifically, the Lim and Ramamoorthy groups). For a detailed summary of our current work in these areas please see our publication list.
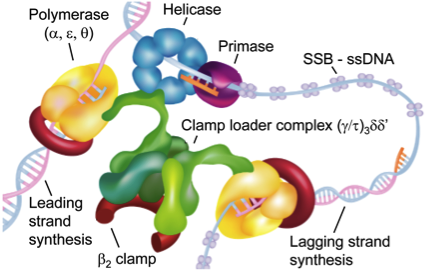
The chromosomal replication machinery from Escherichia coli, known as the DNA replisome, is a dynamic multi-protein machine composed of multiple stable subcomplexes - including the polymerase core (the α core and accessory factors ε and Θ), the sliding clamp (dimeric β2), and the clamp loader complex (comprised of subunits γ3δδχφ), which together deliver highly processive, accurate, and rapid replication. While we study the bacterial proteins, there are many analogous features between the components described here and the analogous eukaryotic systems. The multi-subunit clamp loader complex is responsible for loading and unloading of the sliding clamp β2, which encircles each DNA strand being replicated and maintains processivity by tethering the polymerase core to its substrate. The clamp loader comprises a central pentameric ring, γ3δδ, accountable for the ATPase catalytic activity, and two accessory proteins, χ and Ψ, which form a tight heterodimer and attach to the pentameric ring by associating with either γ or τ a longer version of the γ protein) and interact with single-stranded DNA-binding proteins, SSB. While much is known about the general architecture of this system, the precise orientation and 3D topology of many of the complexes formed during replication are unknown. For example, the orientation of the psi-chi dimer bound to the intact complex is completely unknown and we are attempting to build models, based on IM-MS data, which elucidate its architecture when bound to the clamp loader. We are also studying the architecture of polymerase proteins bound to the sliding clamp dimer as a means of determining how bypass polymerases can deal with damaged DNA sections rapidly and avoid causing multiple cancer-causing mutations to the DNA strand being replicated.
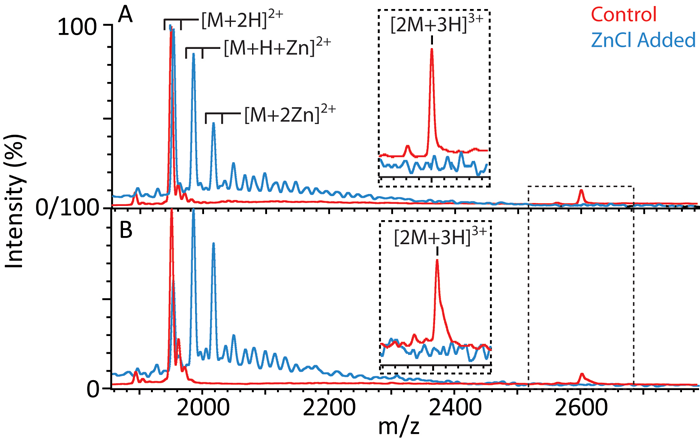
Amyloidosis is a pathogenic form of protein aggregation associated with a number of neurodegenerative diseases. IM-MS has shown tremendous promise as a technology capable of assessing the structure and topology of dynamic, transient multi-protein complexes at low levels. Despite recent successes, there are a number of limitations of IM-MS that have significantly hampered its application to the study of protein aggregation and amyloid formation. Specifically, many aggregation processes take place only at high protein concentrations and in the presence of multiple accessory proteins and metal ions. Both conditions greatly complicate the analysis of IM-MS data. Our approach is to develop new IM-MS data analysis techniques using a novel combination of IM arrival time distribution analysis and a detailed statistical analysis of observed aggregate signals to distinguish between 'gas-phase artifacts' and complexes in solution at any relevant concentration. These analysis techniques will enable us to capture structural information from highly-complex samples and provide the first opportunities to characterize the 3D structure of an extensive and dynamic higher-order protein interaction network surrounding aggregating proteins of previously-unknown structure.
We have begun work in this field by focusing on two amyloid forming systems and their interacting proteins: human amyloid islet polypeptide (hIAPP, type II diabetes) and amyloid β polypeptides (Aβ, Alzheimers disease). Our initial data for hIAPP, shown here, indicates that Zn ions interact with hIAPP in a 2:1 stochiometry and work to suppress dimer formation in the peptide. This loss of dimer population correlates to similar results in vivo that suggest Zn salts suppress fibril formation and islet cell death - indicating that the dimers observed in our experiments could be on the fibril formation pathway for the peptide and a useful, high-throughput diagnostic for the development of therapies for type II diabetes.