Current Research Interests - 2007
Click on the image for a detailed description
Ciliary Transport, Microtubule Organization, and Retinal Dystrophies
 X-linked retinitis pigmentosa (XLRP) is a relatively severe progressive degenerative disease affecting photoreceptors. We have been involved in research on XLRP since 1988 and have a large (perhaps the largest in the world) collection of families and patients with this disease and mapped/identified genetic defects in almost 150 families.
X-linked retinitis pigmentosa (XLRP) is a relatively severe progressive degenerative disease affecting photoreceptors. We have been involved in research on XLRP since 1988 and have a large (perhaps the largest in the world) collection of families and patients with this disease and mapped/identified genetic defects in almost 150 families.
Mutations in RPGR and RP2 account for 80-90% of XLRP. RPGR and RP2 are widely expressed; however mutations in these genes result primarily in photoreceptor cell death. Our studies focus on delineating the function of RPGR and RP2 proteins using biochemical, genetic, cell and molecular biology techniques and by developing mouse models of associated disease.
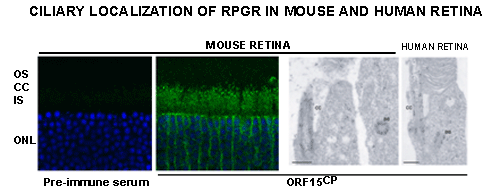
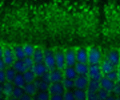
We are also pursuing identification of the molecular lesion in XLRP patients who do not show mutations in the RPGR and RP2 genes. Exciting recent studies suggest novel roles for RPGR and RP2 in microtubule dynamics and/or intracellular trafficking. Our work suggests that RPGR regulates ciliary transport processes in photoreceptors and other cell-types by orchestrating the assembly and trafficking of multi-protein complexes. We have demonstrated that RPGR associates with ciliary transport assemblies in retinal cilia.
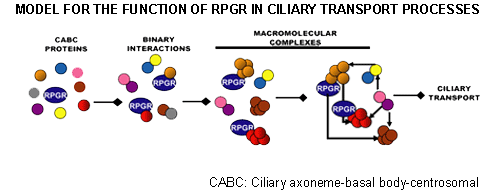
We have also shown that RPGR interacts with the centrosomal-ciliary protein CEP290/NPHP6. In-frame deletion in CEP290/NPHP6 in the rd16 mouse affects its interaction with RPGR and results in early-onset, severe retinal degeneration due to ciliary dysfunction. Loss-of-function mutations in CEP290/NPHP6 are associated with Joubert Syndrome. Our studies assisted in the identification of hypomorphic CEP290/NPHP6 mutations in >20% of LCA. Using yeast two-hybrid strategy, co-immunoprecipitation and mass-spectrometry, our lab has discovered several novel interactors of RPGR and RP2.
We are also examining spontaneously occurring mouse models of XLRP for possible mutations in Rpgr and Rp2. In addition, we are generating mouse models of XLRP by creating conditional null mutations of mouse Rpgr and Rp2 in retinal photoreceptors or RPE using the Cre-loxp strategy (complete knockout has not been possible as yet). The long-term objectives of these studies are to design gene- or pathway-based treatment strategies.