Single molecule Studies of Flavoproteins
Background
Oxygen is a powerful and versatile oxidizing agent that can be used
to create a wide variety of chemical structures. However,
oxygen’s very versatility is also a danger to the cell as oxidation
reactions are generally irreversible and side reactions can generate
toxic byproducts, such as hydrogen peroxide and free radicals. Free
radicals in turn have been implicated in a wide variety of aging
related disorders. In order to avoid the improper oxidation of
substrates oxygenases must have control mechanisms to ensure that
oxygen reacts only under controlled conditions.
The family of flavoprotein monoxygenases represented by
p-hydroxybenzoate hydroxylase (PHBH) offer insight into how such
controlled is obtained. PHBH is a homodimer with two identical subunits
each non-covalently binding one molecule of FAD in the active site. The
environment of the enzyme surrounding the flavin in PHBH is crucial to
the efficiency and regulation of the reaction. The flavin C4
(a)-hydroperoxide, the key intermediate in the catalytic cycle, is
unstable in protic solvents and must be shielded from solvent during
this phase of the reaction to avoid the formation of toxic hydrogen
peroxide. However, the active site must be exposed to solvent in order
for the substrate to bind. A different environment around the active
site is therefore required for each step of the reaction. In addition,
PHBH must distinguish between similar substrates as 4-aminobenzoate, a
close chemical analogue of p-hydroxybenzoate, is an important
intermediate in folic acid synthesis and must not be reduced by the
enzyme.
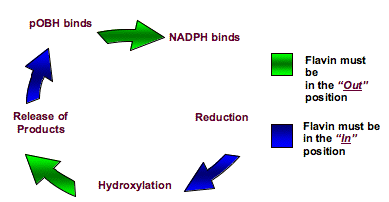
Crystallographic studies on PHBH have suggested that PHBH achieves
this control over the reaction environment by the flavin adopting
different conformations in each step of the reaction. The crystal
structure of PHBH shows the isoalloxazine ring of the flavin in three
conformations depending on the crystallization conditions. When the
wild type protein is crystallized with its natural substrate,
p-hydroxybenzoate, the isoalloxazine ring is buried in the inside of
the protein and is shielded from solvent (the “in” conformation of the
flavin). Under other conditions the flavin crystallizes in what is
called the “out” conformation where the isoalloxazine ring of the
flavin is exposed to solvent and tilted away from the substrate binding
site towards the surface of the protein.
Figure 1.