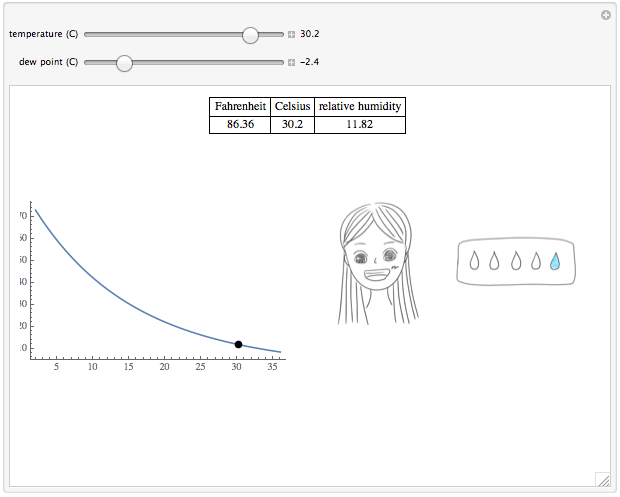
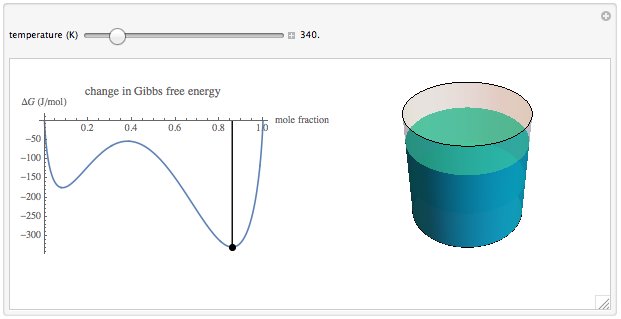
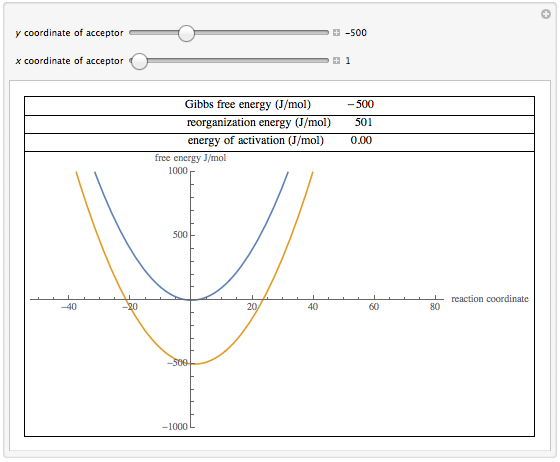
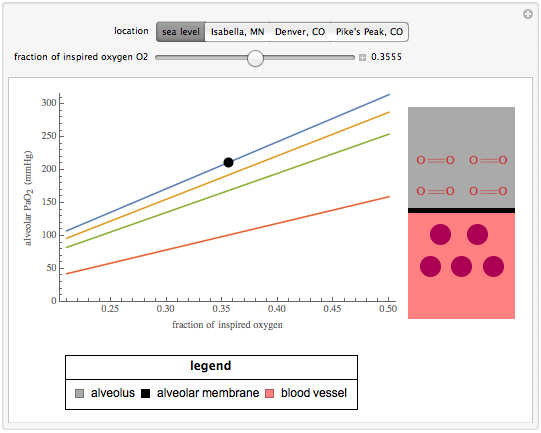
Wolfram Demonstrations Project
Isobaric Compression and Expansion of an Ideal Gas
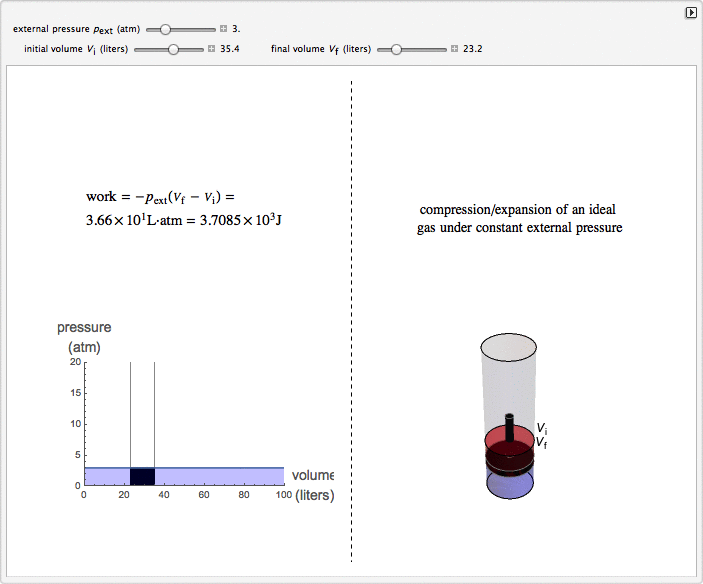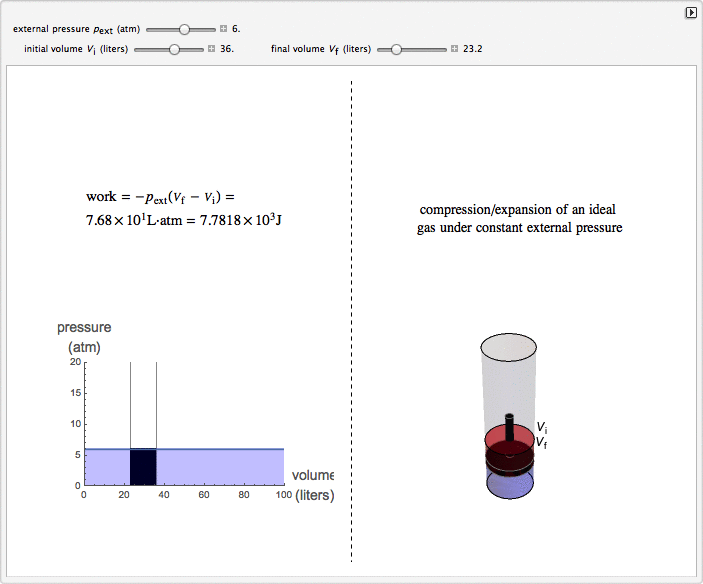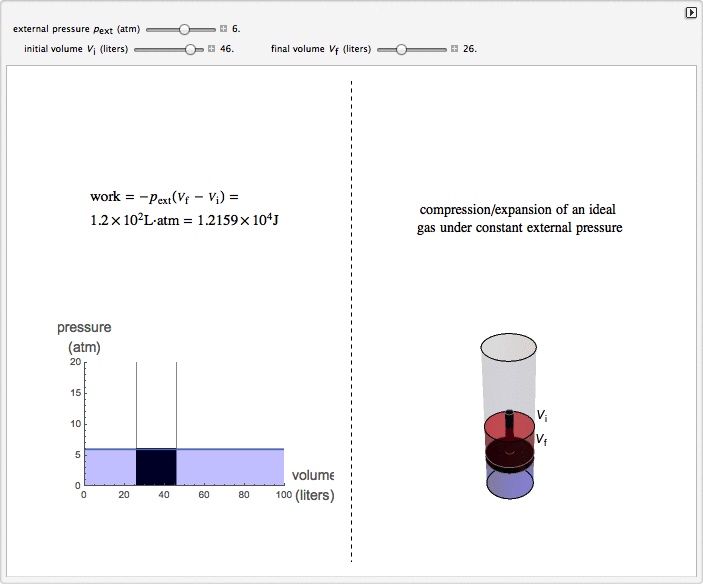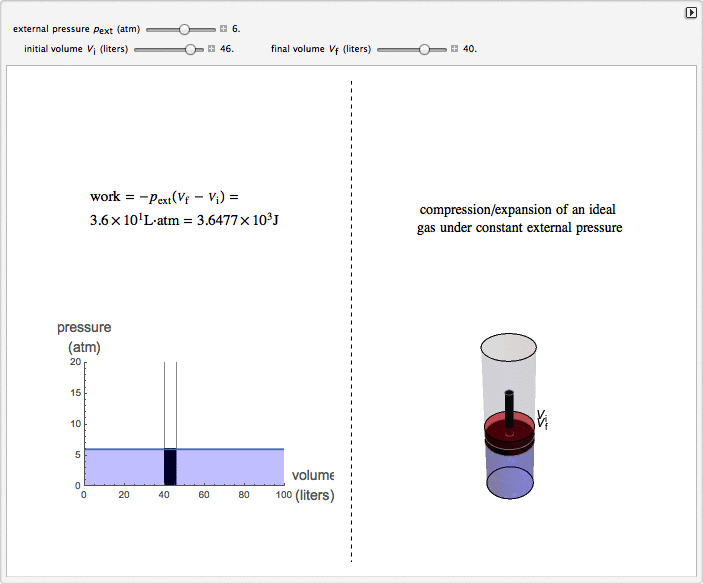
This Demonstration considers the isobaric thermodynamic process of compressing or expanding an ideal gas. Assume that a constant external pressure acts on the piston containing the gas. As you vary the external pressure, the horizontal line in the graph changes accordingly. When the variables for the initial and final volumes are changed, the position of the gridlines on the graph and the initial and final position of the head of the piston move. Work is calculated in the Demonstration with the convention used in chemistry: when the ideal gas expands, the work done is negative (i.e. the ideal gas "loses" energy), and vice versa.
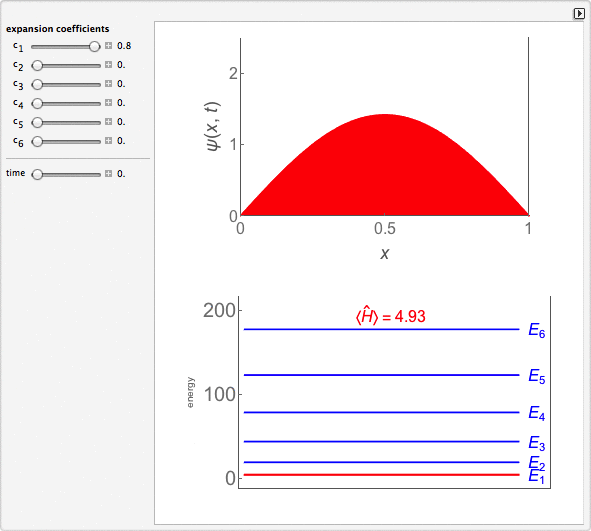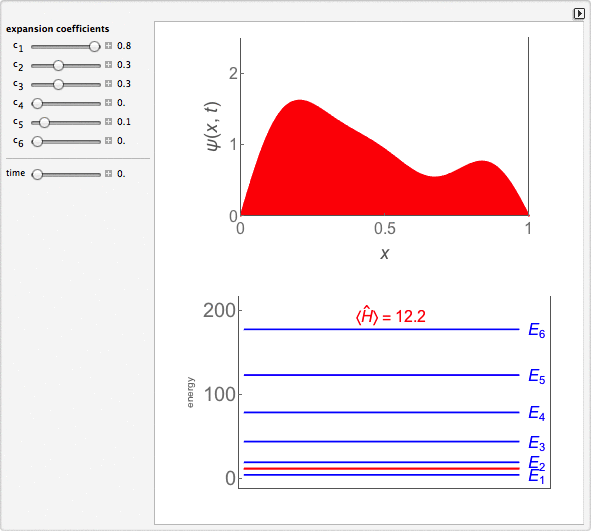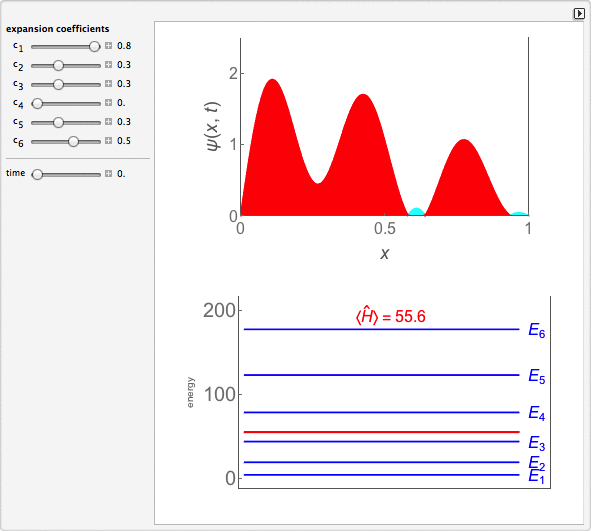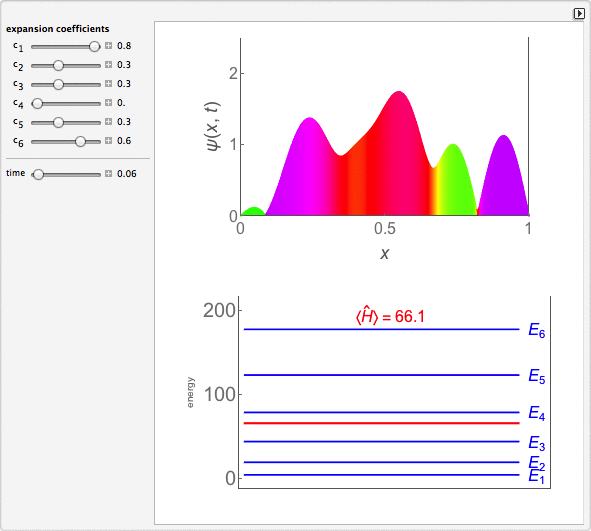
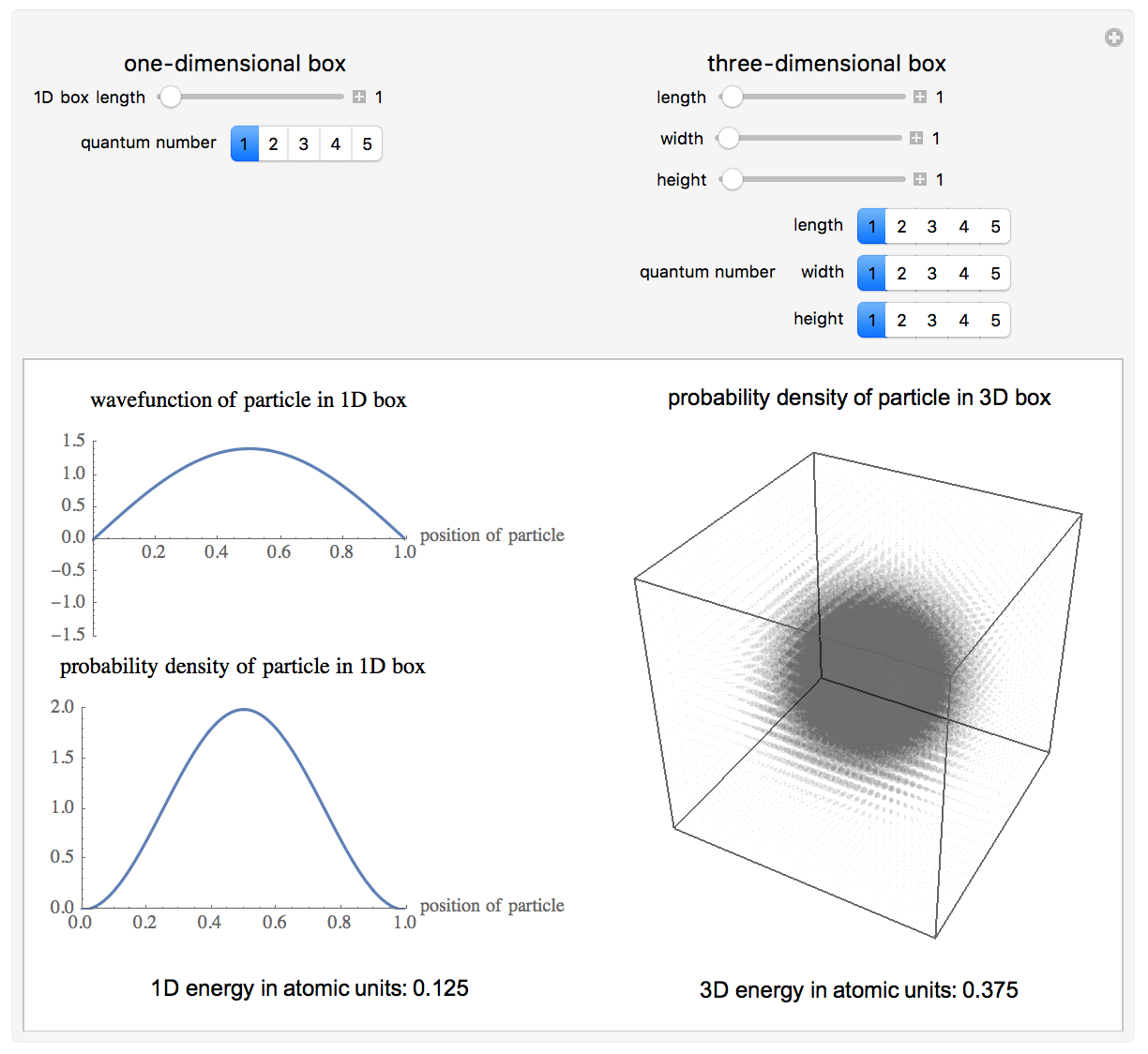
Time-Dependent Superposition of PIAB Eigenstates
This Demonstration looks at a time-dependent superposition of quantum particle-in-a-box eigenstates. The upper panel shows the complex wavefunction, where the shape is its modulus and the coloring is according to its argument (the range 0 to 2π corresponds to colors from red to magenta). The lower panel shows the eigenenergies in blue and the energy of the superposition state in red.