|

|
Binding Studies using 1H-NMR
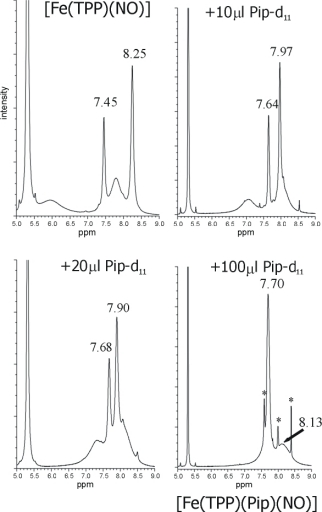
NMR titration of [Fe(TPP)(NO)] (1, top left) with piperidine-d11 (Pip) yielding
[Fe(TPP)(Pip)(NO)] (bottom right) showing a clean conversion of the five-coordinate (5C) to the
six-coordinate (6C) complex. The small diamagnetic signals appearing in the product spectrum
(bottom, right; marked with an asterisk) are due to formation of [Fe(TPP)(Pip)2]
following the general equation:

(here, L = Pip).
This is an important point, because it shows that the large concentrations of base often used for the
formation of the six-coordinate complexes [Fe(TPP*)(L)(NO)] in solution lead to denitrosylation,
and hence, loss of NO. This problem has been mostly overlooked in the literature for two reasons.
Firstly, the electronic absorption spectra of [Fe(TPP*)(L)(NO)] and [Fe(TPP*)(L)2]
are usually very similar. Solution EPR and IR measurements clearly show that [Fe(TPP*)(L)(NO)] is formed upon
addition of base to [Fe(TPP*)(NO)], but [Fe(TPP*)(L)2] is not detected in these
experiments because it is EPR silent and does not have characteristic IR bands.
We found that 1H-NMR spectroscopy is a very useful tool to monitor the formation of even small amounts
of [Fe(TPP*)(L)2] in solution as indicated in the top Figure.
|
