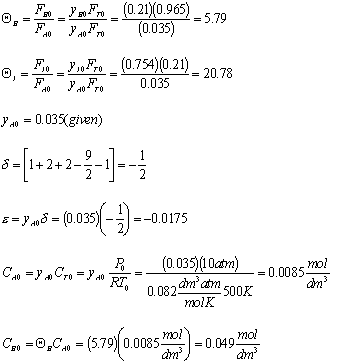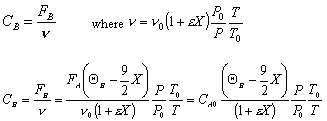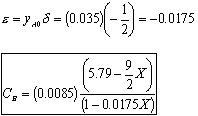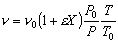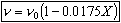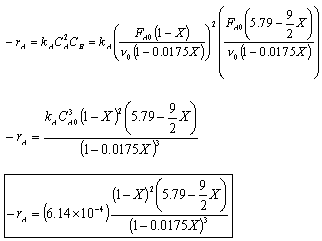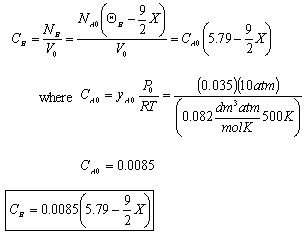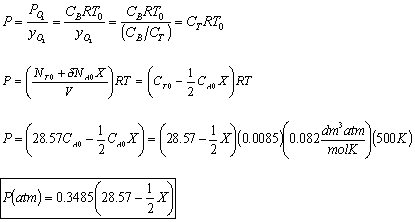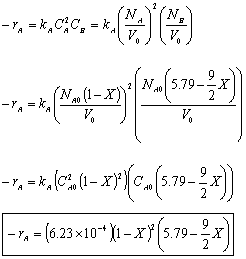Chapter 3 Example
Oxidation of Naphthalene to Phtahalic Anhydride
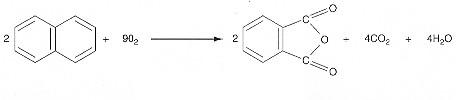
Taking A as the basis of calculation, A + 9/2 B → C + 2D + 2E
We are going to rework the class problem for the case of 3.5% naphthalene and 96.7% air and for a pressure of 10 atm and a temperature of 500K. A feed under these conditions naphthalene is the limiting reactant.
- Stoichiomentric Table - Batch System
| Species |
Symbol |
In |
Change |
Out |
| Naphthalene |
A |
NA0 |
-NA0X |
NA = NA0(1-X) |
| Oxygen |
B |
NB0 = 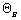 NA0 NA0 |
-9/2NA0X |
NB = NA0(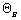 - 9/2X) - 9/2X) |
| Phthalic Anhydride |
C |
NC = 0 |
+NA0X |
NC = NA0X |
| Carbon Dioxide |
D |
ND = 0 |
+2NA0X |
ND = 2NA0X |
| Water |
E |
NE = 0 |
+2NA0X |
NE = 2NA0X |
| Nitrogen |
I |
NI = 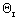 NA0 NA0 |
--- |
NI = 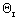 NA0 NA0 |
| Total |
|
NT0 |
|
NT = NT0 + 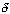 NA0X NA0X |
| |
|
|
|
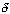 = [1 + 2 + 2 - 9/2 - 1] = -1/2 = [1 + 2 + 2 - 9/2 - 1] = -1/2
|
Stoichiometric Table - Flow System
| Species |
Symbol |
In |
Change |
Out |
| Naphthalene |
A |
FA0 |
-FA0X |
FA = FA0(1-X) |
| Oxygen |
B |
FB0 = 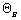 FA0 FA0 |
-9/2FA0X |
FB = FA0(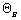 - 9/2X) - 9/2X) |
| Phthalic Anhydride |
C |
FC = 0 |
+FA0X |
FC = FA0X |
| Carbon Dioxide |
D |
FD = 0 |
+2FA0X |
FD = 2FA0X |
| Water |
E |
FE = 0 |
+2FA0X |
FE = 2FA0X |
| Nitrogen |
I |
FI = 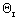 FA0 FA0 |
--- |
FI = 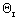 FA0 FA0 |
| Total |
|
FT0 |
|
FT = FT0 + 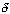 FA0X FA0X |
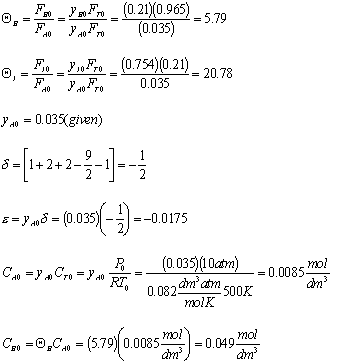
- Determine each of the following soley as a function of the conversion of naphthalene, X for a constant-pressureisothermal flow reactor.
- Find the concentration of O2
Gas Phase Flow System:
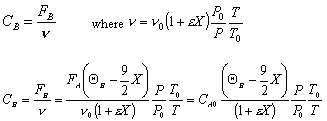
Constant pressure and isothermal: P = Po; T = To
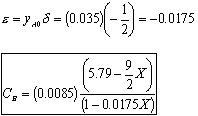
- Find the volumentric flowrate
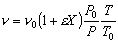
Constant pressure and isothermal: P = Po; T = To
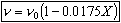
- Find the reaction rate
Rate Law: -rA = kAC2ACB
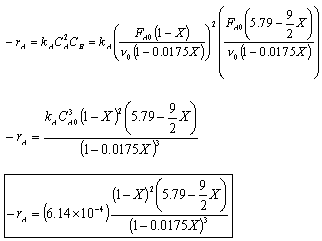
For a constant volume isothermal batch reactor,
- The concentration of O2
For Batch: V = V0, CB = NB/V = NB/V0
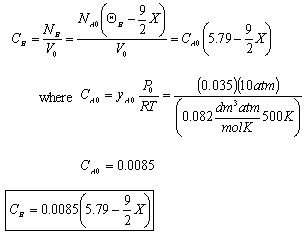
- The total pressure, P.
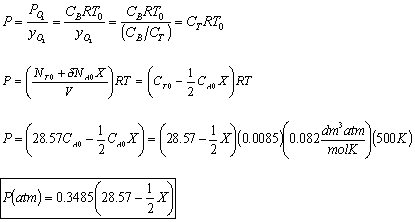
- The rate law
Rate Law: -rA = kAC2ACB
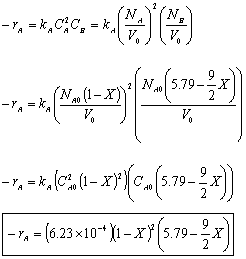
Back to Chapter 3
 NA0
NA0 - 9/2X)
- 9/2X)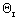 NA0
NA0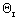 NA0
NA0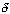 NA0X
NA0X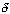 = [1 + 2 + 2 - 9/2 - 1] = -1/2
= [1 + 2 + 2 - 9/2 - 1] = -1/2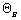 FA0
FA0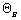 - 9/2X)
- 9/2X)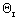 FA0
FA0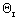 FA0
FA0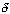 FA0X
FA0X