The elementary irreversible gas phase catalytic reaction
![]()
is carried out isothermically in a batch reactor. The catalyst deactivation follows a first order decay law and is independent of the concentrations of both A and B.
Additional information:
CA0 = 1 mol/dm3
V0 = 1 dm3
W = 1 kg
kd = 0.1 min-1 at 300 K Ed/R = 2,000 K
k1 =0.2 dm3/(kg cat · min) at 300 K Ea/R = 500 K
Hint 1 Write the algorithm and determine a general expression for catalyst activity as a function of time.
Hint 2 Make a qualitative sketch of catalyst activity as a function of time. Does a(t) ever equal zero for a first order decay law?
Hint 3 Write out the general algorithm and derive an expression for conversion as a function of time, the reactor parameters and the catalyst arameters.
Fill in the following algorithm
Mole balance
Rate Law
Decay Law
Stoichiometry
Combine
Solve
1) Separate
2) Integrate
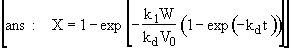
Hint 4 Calculate the conversion and catalyst activity in the reactor after 10 minutes at 300 K.
Hint 1. Algorithm and a(t)
Batch–
Mole Balance
![]()
Rate Law
![]()
Decay Law
First-order decay law
|
|
|
|
Hint 2
Sketch a(t)
|
|
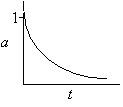
|
a(t) never equals zero for a first-order decay law |
Hint 3. Determine X(t)
Stoichiometry
![]()
Combine
![]()
Separate and Integrate
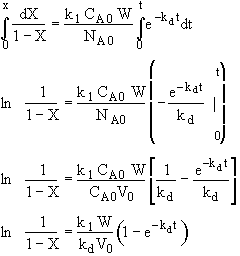
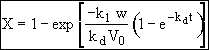
where
![]()
![]()
Hint 4
Calculate conversion after 10 minutes
300 K – No temperature correction needed for k1, kd since T = T1
Results the same for both catalysts
![]()
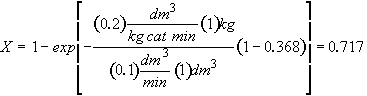
For both catalysts 1 and 2 at 300 K
Hint 1. Algorithm and a(t)
Batch–
Mole Balance
![]()
Rate Law
![]()
Decay Law
First-order decay law
|
|
|
|
Hint 2
Sketch a(t)
|
|

|
a(t) never equals zero for a first-order decay law |
Hint 3. Determine X(t)
Stoichiometry
![]()
Combine
![]()
Separate and Integrate
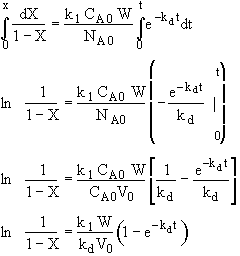
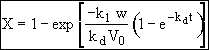
where
![]()
![]()
Hint 4
Calculate conversion after 10 minutes
300 K – No temperature correction needed for k1, kd since T = T1
Results the same for both catalysts
![]()
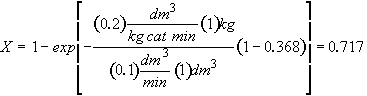
For both catalysts 1 and 2 at 300 K