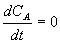
In some chemistry books the rate of reaction has been defined as
The mathematical definition of a chemical reaction rate has been a source of confusion in chemical and chemical engineering literature for many years. The origin of this confusion stems from laboratory bench-scale experiments that were carried out to obtain chemical reaction rate data. These early experiments were batch type, in which the reaction vessel was closed and rigid; consequently, the ensuing reaction took place at constant volume. The reactants were mixed together at time t = 0 and the concentration of one of the reactants, CA, was measured at various times t. The rate of reaction was determined from the slope of the plot of CA as a function of time. Letting rA be the rate of formation of A per unit volume (e.g. mol/s•dm3), the investigators then defined and reported the chemical reaction rate as
rA= dCA/dt (1-1)
However this "definition" is wrong! It is simply a mole balance that is only valid for a constant volume batch system. Equation (1-1) will not apply to any continuous-flow reactor operated at steady state, such as the tank (CSTR) reactor where the concentration does not vary from day to day (i.e., the concentration is not a function of time).
Sodium hydroxide and ethyl acetate are continuously fed to a rapidly stirred tank in which they react to form sodium acetate and ethanol:
NaOH + CH3COOC2H5 ----> CH3COONa + C2H5OH
(Figure E1-1.1). The product stream, containing sodium acetate and ethanol, together with the unreacted sodiium hydroxide and ethyl acetate, is continuously withdrawn from the tank at a rate equal to the total feed rate. The contents of the tank in which this reaction is taking place may be considered to be perfectly mixed. Because the system is operated at steady state, if we were to withdraw liquid samples at some location in the tank at various times and analyze them chemically, we would find that the concentrations of the individual species in the different samples were identical. That is, the concentration of the sample taken at 1 P.M. is the same as the that of the sample taken at 3 P.M. Because the species concentrations are constant and therefore do not change with time,
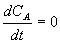
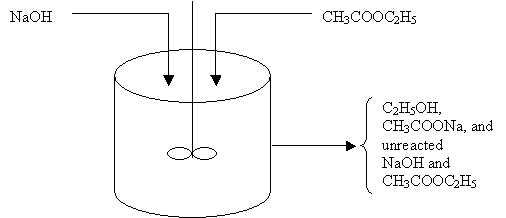
Figure E1-1.1 Well mixed reaction vessel
Where A is NaOH. Substitution of Equation (E1-1.1) into Equation (1-1) leads to
rA = 0
Which ia incorrect because C2H5OH and CH3COONa are being formed from NaOH and CH3COOC2H5 at a finite rate. Consequently, the rate of reaction as defined by Equation (1-1) cannot apply to a flow system and is incorrrect if it is defined in this manner. rA is an algebraic law!