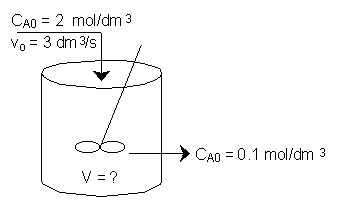
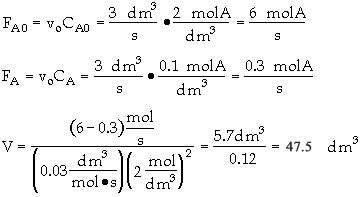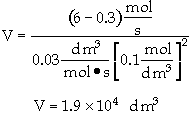The irreversible liquid phase second order reaction
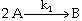
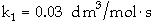
is carried out in a CSTR.
The entering concentration of A, CA0, is 2 molar and the exit concentration of A, CA is 0.1 molar.
The entering and exiting volumetric flow rate, vo, is constant at 3 dm3/s.
What is the corresponding reactor volume?
The entering concentration, CAO = 2 mol/dm3 of A was substituted into the rate law instead of the exit concentration CA = 0.1 mol/dm3.
Because the CSTR is perfectly mixed, the concentration inside the CSTR where the reaction is taking place is the same as that in the exit.
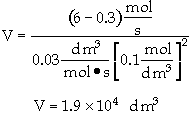
The reactor volume is fairly large at about 5 thousand gallons.
(3.785 dm3 = 1 US gallon)
Back to Chapter 1
![]()
![]()