Determine the molar flux of ammonia, WA,and the mass flux of ammonia, mA,through the stationary frame at steady state for the system shown in Figure CDE11-1.1. Ammonia is diffusing at steady state from point 1 to point 2. There are 12.04 x 1023 molecules of ammonia that pass through an area bounded by a stationary frame with a dimension of 1 m x 2 m in the direction of the lower concentration and over a time interval of 10 s.
Solution
The flux is the flow rate per unit area normal to the direction of flow. The number of moles of ammonia crossing the boundary in the 10-s interval is
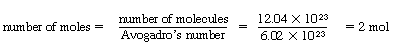

Figure CDE11-1.1
Two moles pass through an area of 2 m2 in 10 s. Therefore, the number of moles passing through 1 m2 per second is
![]()
(CDE11-1.1)
The molar flow rate is

(CDE11-1.2)
The mass flux of A is the molar flux of A times the molecular weight of A, MA:

(CDE11-1.3)