 ) of the solution from which the
ferricyanide was omitted. The solution compositions were:
) of the solution from which the
ferricyanide was omitted. The solution compositions were:The molecular diffusivity of potassium ferricyanide in a solution was determined by filling a capillary with the
solution and immersing it in a bath (at 25  ) of the solution from which the
ferricyanide was omitted. The solution compositions were:
) of the solution from which the
ferricyanide was omitted. The solution compositions were:
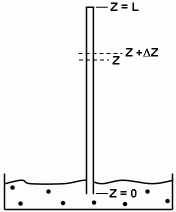
The capillary tube is 1.0 mm in diameter and 2.1 cm long. After 15 hours the capillary was removed from the bath and its contained fluid titrated for ferricyanide. It was found to contain 2.308 micro-moles of potassium ferricyanide.
 ?
? ?
?(Derive the differential equation and boundary conditions for this process and put the problem in dimensionless form. The error function solution may then be written down, if known.)
Let: A = potassium ferricyanide
B = all other components
Initial condition
t = 0, CA = 0, Z 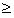 0
0
Boundary Conditions

A mass balance on component "A" in the capillary element S Z gives:
Z gives:

Dividing by S Z and taking the limit
Z and taking the limit

Fick's first law for dilute solutions is

from which

Thus we arrive at Fick's second law in the form

Utilizing:


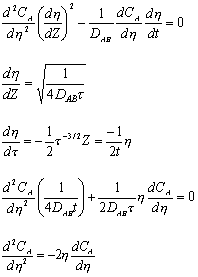



 )
)The following solution results

differentiating for (dCA/dZ) and substituting

evaluating @ Z = 0

for the period of the experiment, the weight loss (M)


Rearranging and evaluating

To evaluate the error introduced by assuming Z  utilize
utilize

|
|
|
0.9999 |
2.0 |
2.17 |
0.99 |
1.81 |
1.97 |
Thus (0.99 < 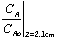 < 0.9999) @ t = 15 hr.,
< 0.9999) @ t = 15 hr.,
the assumption (Z  ,
,
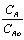 = 1.0) was pretty good.
= 1.0) was pretty good.
For liquid diffusivity

Assuming for this dilute solution


