
Dissolution of Pills in the Stomach
Human studies have shown that the absorption of griseofulvin increased as the particle size decreased which was predicted by the model. This same study, which included particle size distribution as well, reported that there was no significant difference between two particle populations. However, the distributions were defined only as “narrow” or “wide” and therefore the results cannot be compared to the simulations of the model. Since all particles in both ranges were < 10mm, if the actual increase in geometric standard deviation was small, then a difference in the fraction dose absorbed may also be small. However, if the distribution is wide the results may be uncertain and needs to be analyzed. A second study showed that a diet of greasy fried foods and nuts increased the absorption of griseofulvin significantly. This increase may be a result of delayed gastric emptying and therefore a smaller Dose Number resulting in a predicted increased in absorption.
Most of the variations in absorption of carbarnzepine in humans have been reported as clinical observations. Because carbarnzepine is used primarily for seizure control in epileptics, an, increase in the number of seizures has been reported when the patient switches from one company's, product to another. Amidon’s studies indicate that large differences in the intestinal absorption can result from small differences in the particle size range even if the mean particle size is constant. Consequently, an understanding of the dissolution of particles must be undertaken. [G. L. Amidon and J. Cusion, Pharm. Res. S – 170 (Nov. 1992) San Antonio TX.]
In the analysis below we consider the dissolution of capsules (pills) in a fluid (stomach) when there is a distribution of capsule sizes. We want to determine how the distribution changes as the particles (pills/capsules) dissolve. We model the system as a solid B dissolving in a liquid A.

Reactant A in the liquid phase (say a stomach acid) diffuses to the solid surface where it reacts on the surface.

At steady state the rate of reaction of A on the surface is equal to the rate of external diffusion of A to the surface. For a first order surface reaction
 (1)
(1)
Solving for CAS
 (2)
(2)
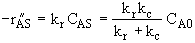
The mass transfer coefficient is given by the Frössling correlation for the Sherwood number, Sh
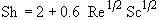
in terms of the Reynolds, (Re) and Schmidt (Sc) numbers.
For small particles: Sh = 2
then

also




Let

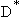 = Particle diameter at which mass
transfer and reaction rate resistances are equal
= Particle diameter at which mass
transfer and reaction rate resistances are equal
D > Mass Transfer Controls
Mass Transfer Controls
D <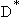 Reaction Rate Controls
Reaction Rate Controls
The concentration profiles at any time t are given by the steady state profiles.
(Pharmacokinetics) Pills in the stomach.




where rB is the density of solid B
 and the stoichiometric coefficient,
and the stoichiometric coefficient, , is taken as unity, but any other value will not change the analysis
, is taken as unity, but any other value will not change the analysis

For X. S. Acid
CA ~ Constant = CA0

At t = 0, D = Di
where


Time to complete dissolution

Suppose the pills are small and encapsulated in a gelatin capsule similar to ContactTM cold medication and the pills are slightly different diameters


F(D) DD = no. of particles with diameters between D and D + DD
The initial distribution of particle sizes is given by log-normal distribution

where N0 is the number of particles initially, Dg and s2 are parameters of the distribution of the specified particle
Population balances



Taking the limit as DD ® 0
Where R(D) is the growth rate of particles of size D, e.g. (cm/min).
[Note:
R(D) º
R and F(D) º
F]

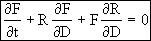


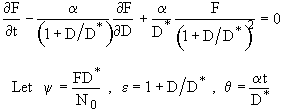

This equation is an ODE of the form
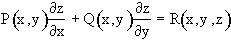
and can be solved by the method of characteristics

Recall applied to our equation

from

A solution will be in the form

Now from


The solution will be of the form of e times some function, H, of (e2 + 2q)

The initial distribution

Rearranging in terms of e



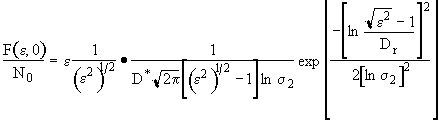
Now everywhere we see e2 we replace it by e2 + 2q.

In dimensional form


|
