


(P. E. Savage, The University of Michigan)
State explicitly any further assumptions you make. Assume that the oxide
layer has some initial thickness T0
at time t = 0.
[2nd Ed. P10-17]
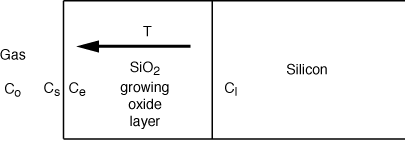
| The oxidation of silicon to form
SiO2 is an important step in the fabrication
of microelectronic devices. The oxidation process can be modeled by three
events: (1) the diffusion of oxidant from the bulk gas to the surface of
the oxide layer, (2) the diffusion of oxidant through the growing oxide
layer to the Si-SiO2 interface, and (3)
the reaction of oxidant with Si at the Si-SiO2 interface [J. Appl. Phys. 36,
3770 (1965)]. Refer to the sketch below for additional details.
|

|
| The reaction at the Si surface is assumed to be first-order
in oxidant and zero-order in Si. Derive an expression for the oxide layer
thickness as a function of time, and show that it can be written in the
following form:
(P. E. Savage, The University of Michigan) State explicitly any further assumptions you make. Assume that the oxide
layer has some initial thickness T0
at time t = 0. |