| |
|
|
|
| |
|
The approximate error in the correlation is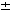 20%.
Other limitations of the correlation can be found in the article just cited
by Arashi et al.1 20%.
Other limitations of the correlation can be found in the article just cited
by Arashi et al.1
For no volume change with reaction, Equation (R11.1-4) can be integrated
to give |
|
| |
|
|
|
| |
|

|
(R11.1-7) |
| |
|
|
|
|
Ford and Chrysler
use monolith catalytic
afterburners.
|
|
A variation of the monolith reactor has the gas flowing through square
(or other shape) channels as shown in Figure R11.1-3. This reactor is also
known as a honeycomb reactor. Monolith reactors are used as catalytic
afterburners on automobiles and are manufactured by Chrysler and Ford.2
| |
| |


(b)
Figure R11.1-3
(a) Honeycomb reactor; (b) catalytic afterburner. (Photo courtesy of Engelhard Corporation)
| |
| |
|
|
|
| |
|
B. Wire Gauzes Wire gauzes are commonly used in the
oxidation of ammonia and hydrocarbons. A gauze is a series of wire screens,
stacked one on top of another (Figure R11.1-4). The wire is typically made
out of platinum or a platinum-rhodium alloy. The wire diameter ranges between
0.004 and 0.01 cm.
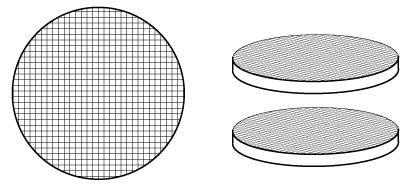 Figure R11.1-4
Figure R11.1-4
Wire gauzes.
As a first approximation, one can assume plug flow through the gauze, in which case
the design equation is similar to that for monolith reactors,
|
|
| |
|
|
|
|
Differential form
of the wire gauze
design equation
|
|

|
(R11.1-8) |
| |
|
|
|
| |
|
where ag = total screen
surface area per total volume of one screen, m2/m3
or in2/in3
n = number of screens in series
V = n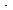 (volume
per screen) (volume
per screen)
The values of ag can be
calculated from the equations3

|
|
| |
|
where d = wire diameter, in.
N = mesh size,
number of wires per linear inch
In calculating the volume of the screen, the thickness is taken as twice
the wire diameter (i.e., 2d). The porosity can be calculated from
the equation |
|
| |
|
|
|
| |
|

|
|
| |
|
|
|
| |
|
The mass transfer coefficient can be obtained from the correlation
for one to three screens, |
|
| |
|
|
|
| |
|
|
|
| |
|
For one to five screens, the correlation is |
|
| |
|
|
|
| |
|
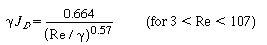
|
(R11.1-11) |
| |
|
|
|
| |
|
where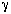 is
the minimum fractional opening of a single screen: is
the minimum fractional opening of a single screen: |
|
| |
|
|
|
| |
|
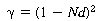
|
(R11.1-12) |
| |
|
|
|
| |
|
In the commercial process for the oxidation of ammonia, typical
parameter values are conversion
of ammonia. conversion
of ammonia.
|
|
| |
|
When more than one or two screens are necessary, some backmixing
takes place. Shimizu et al.
4 account for this backmixing by introducing dispersion
in the axial direction: |
|
| |
|
|
|
| |
|

|
(R11.1-13) |
| |
|
|
|
|
Too few screens?
|
|
Equation (R11.1-13) is then combined with Equation (R11.1-8)
and solved. When dispersion is significant it was shown that, depending
on the flow conditions, 33 to 300% more screens were required than predicted
by the plug-flow model. |
|
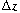 (Figure
R11.1-2).
(Figure
R11.1-2). 



 0
yields
0
yields



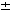 20%.
Other limitations of the correlation can be found in the article just cited
by Arashi et al.
20%.
Other limitations of the correlation can be found in the article just cited
by Arashi et al.




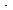 (volume
per screen)
(volume
per screen)



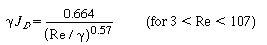
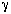 is
the minimum fractional opening of a single screen:
is
the minimum fractional opening of a single screen: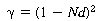
 conversion
of ammonia.
conversion
of ammonia.