The gas-phase cracking of a Salina light gas-oil reaction
A typical cost
of the catalyst
is
$1 million
gas-oil (g)  products (g) + coke (s)
products (g) + coke (s)
A  B
B
is to be carried out in a straight-through transport reactor containing
a catalyst that decays as a result of coking. The reaction is carried out at 750°F.
The entering concentration of A is 0.2 kmol/m 3 .
The catalyst particles are assumed to move with the mean gas velocity (U g =U s
= 7.5 m/s). The rate law is
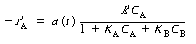
with K A = 3 m 3 /kmol and K
B = 0.01 m 3
/kmol. The maximum value of the term K B
C B is
small (0.002), so it can be neglected with respect to the other terms (e.g., 1).
Using this fact and the bulk density,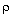 b
, the rate law becomes
b
, the rate law becomes
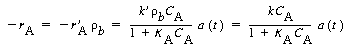
with k = 8 s -1 . At 750°F, the catalyst activity for light gas-oil over a synthetic catalyst for short contact times (i.e., less than 100 s) is
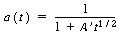
with A ´= 7.6 s -1/2 .
As a first approximation, neglect volume change with reaction, pressure drop, and
temperature variations.
Determine the conversion as a function of distance down the reactor. The reactor
length is 6 m.
Solution
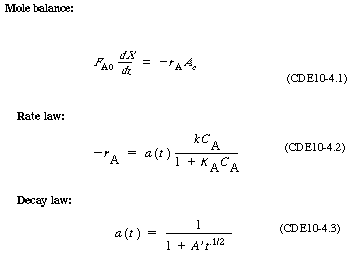
For a catalyst particle traveling with a velocity of U, the
time that the catalyst particle has been in the reactor when it reaches a height is just
is just
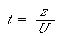
(CDE10-4.4)
and
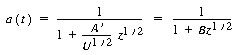
(CDE10-4.5)
where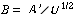
Stoichiometry. Gas-phase reaction with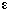 = 0,
T = T 0 , and P = P 0.
= 0,
T = T 0 , and P = P 0.
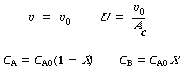
Combining yields
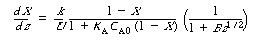
(CDE10-4.6)
POLYMATH Solution. In using POLYMATH to obtain a solution, it is usually easiest to write the mole balance, rate law, and stoichiometry separately rather than combining them into a single equation. Therefore, starting with the mole balance
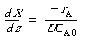
(CDE10-4.7)
we substitute for -r A and a using Equations (CDE10-4.2) and (CDE10-4.5). The POLYMATH program and solution are shown in Table CDE10-4.1 and Figure CDE10-4.1 respectively.
Table CDE10-4.1
POLYMATH Program Coking in a Straight-Through Transport Reactor
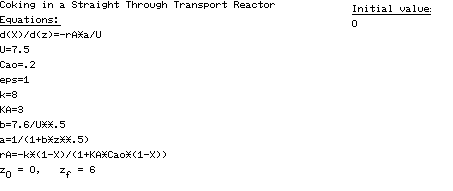
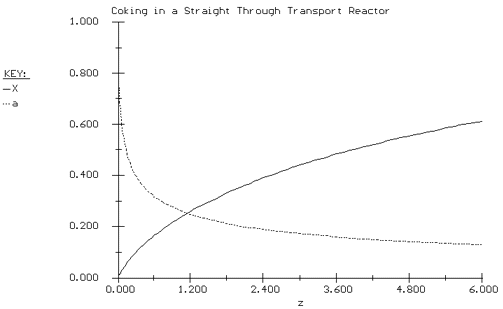
Figure CDE10-4.1
Conversion and Activity Profiles