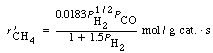
It is desired to produce 20 tons/day of CH 4 . Calculate the catalyst weights necessary to achieve 80% conversion in:
(a) A fixed bed.
(b) A fluidized bed.
The feed consists of 75% H 2 and 25% CO at a temperature of 500°F and a pressure of 10 atm. Assume both molecular and atomic hydrogen are adsorbed on the surface.