| The dehydrogenation of ethylbenzene is one of the most important methods
for the manufacture of styrene. The reaction has been studied using a Shell 105 catalyst
(93 wt % Fe2 O 3
, 5% Cr 2 O
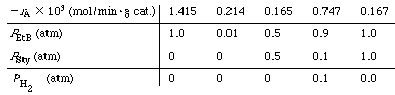
3 , 2% KOH) in a differential reactor [Ind. Eng. Chem. Process Des. Dev. 4, 281 (1965)]. It was observed that the rate of reaction was decreased when styrene was added to the feed stream. Initial rate data showed that as the partial pressure of ethylbenzene was increased to moderate values, the rate of reaction became independent of the partial pressure of ethylbenzene. However, the rate of reaction was also decreased when styrene and hydrogen were formed. The equilibrium constant K p was taken to be 0.415 atm at 630°C. Suggest a mechanism that is consistent with the experimental observations and derive the corresponding rate law. Using the information from the following table, taken at T = 630°C |
||
|
|
|
evaluate all constants in your model and the determine the total cost of catalyst necessary to produce 2000 kg of styrene per day in (a) A CSTR fluidized-bed reactor. [2nd Ed. P6-20] |