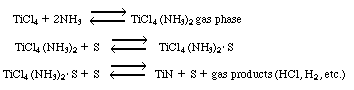
| Titanium nitride films are used in decorative coatings as well as
in wear-resistant tools. There is increasing interest in TiN because of its thermal
stability, good diffusion barrier properties, and its low electrical resistivity [J. Electrochem. Soc., 138, 500 (1991)]. Titanium nitride films were formed by CVD from a mixture of TiCl 4 , NH 3 , H 2 and Ar. The following observations can be made from the article: |
||
|
||
|
|
|
It is believed that the gas-phase reaction to form the complex TiCl4 (NH 3 ) 2 is in equilibrium. (a) Determine the rate expression for the suggested mechanism.
Does it agree with experimental observations? |
||
|
|