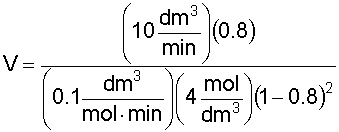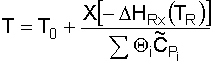Part A
Second Order Reaction Carried Out Adiabatically in a CSTR(a) We will solve part (a) by using the non isothermal
reactor design algorithm discussed in Chapter 8.
1. CSTR Design Equation:
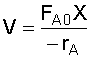
2. Rate Law:
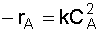
3. Stoichiometry:
liquid,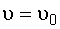
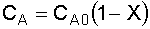
4. Combine:
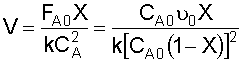
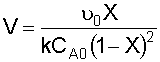
Given conversion (X), you must first determine the reaction temperature (T),
and then you can calculate the reactor volume (V).
5. Determine T:
From the adiabatic energy balance (as applied to CSTRs):
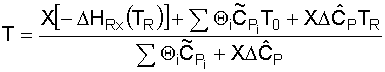
which reduces to:
Substituting for known values and solving for T:
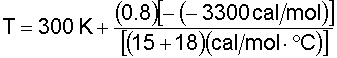
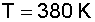
6. Solve for the Rate Constant (k) at T = 380 K:
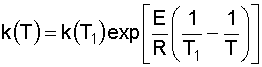
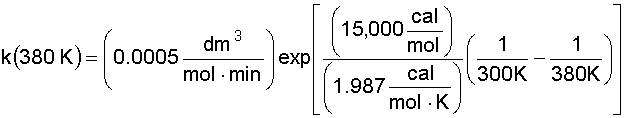
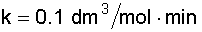
7. Calculate the CSTR Reactor Volume (V):
Recall that:
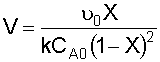
Substituting for known values and solving for V: