Additional Homework Problems
CDP6-24B
The production of maleic anhydride by the air oxidation of benzene was recently studied using a vanadium pentoxide catalyst [Chem Eng. Sci., 43, 1051 (1988)]. The reactions that occur are:

Because these reactions were carried out in excess air, volume change with reaction can be neglected, and the reactions can be written symbolically as a pseudo-first-order reaction sequence

where A = benzene, B = maleic anhydride, C = products (H2O, CO2), D = products (CO2, H2O). The corresponding pseudo specific reaction rates, ki are (in m3/kg cat/s):

At 848 K, k1 = 1.4 X 10-3, k2 = 1.46 X 10-3, k3 = 7.65 X 10-5. These reactions are carried out isothermally in both a CSTR and a PBR. Benzene enters the reactor at a concentration of 0.01 mol/dm3. The total volumetric flow rate is 0.0025 m3/s.
- Which reactions will dominate at low temperatures and which will dominate at high temperatures? For the sake of comparison, assume that 848 K is a moderate temperature.
- For a catalytic weight of 50 kg, determine the exit concentrations from a "fluidized" CSTR at 848 K. (Ans: CB =0.3 mol/dm3
- What is the selectivity of B to C and of B to D in the CSTR?
- Plot the concentrations of all species as a function of PBR catalyst weight (up to 10 kg) assuming isothermal operation at 848 K.
- What feed conditions and reactor or combinations of reactors shown in Figure 6-3 would you use to maximize the production of maleic anhydride?
- How would your results in part (d) change if pressure drop were taken into account with
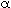 = 0.099 kg cat-1 in the PBR? Make a plot similar to that in part (d) and describe any differences.
= 0.099 kg cat-1 in the PBR? Make a plot similar to that in part (d) and describe any differences.
[3rd Ed. P6-14]



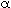 = 0.099 kg cat-1 in the PBR? Make a plot similar to that in part (d) and describe any differences.
= 0.099 kg cat-1 in the PBR? Make a plot similar to that in part (d) and describe any differences.