The elementary reaction
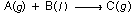
is taking place only in the gas phase of a square duct. The feed to the
duct consists of a gas stream of pure A and a liquid stream of pure B.
The flowing liquid B covers the bottom of the duct and evaporates into
the gas phase, maintaining its equilibrium vapor pressure throughout the
system. The gas phase flows in plug flow. Ignore the volume occupied by
liquid B (see Figure CDP3-C).

(b) What is the rate of reaction, -rA, when the conversion is 50%?
| Total pressure (considered constant): 1 atm Value of k: 106 ft3/lb mol 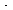 s sTemperature within the reactor (considered constant): 540°F Vapor pressure of B: 0.25 atm at 540°F Inlet flow rate of A: 1.5 lb mol/ s (Ans.: 0.174 lb mol/ ft3 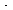 s.) s.) |