 C
CThe irreversible gas phase nonelementary reaction
A+2B C
C
is to be carried out isothermally in a constant-pressure batch reactor. The feed is at a temperature of 227oC, a pressure of 1013 kPa, and its composition is 33.3% A and 66.7% B. Laboratory data taken under identical conditions are as follows (note that at X= 0, -rA=0.00001):
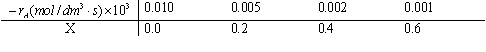
(a) Estimate the volume of a plug-flow reactor required to
achieve 30% conversion of A for an entering volumetric flow rate of 2
m3/min.
(b) Estimate the volume of a CSTR required to
take the effluent from the plug-flow reactor (PFR) above and achieve 50% total
conversion (based on species A fed to the PFR).
(c) What is the
total volume of the two reactors?
(d) What is the volume of a
single plug-flow reactor necessary to achieve 60% conversion? 80%
conversion?
(e) What is the volume of a single CSTR necessary to
achieve 50% conversion?
(f) What is the volume of a second CSTR
necessary to raise the conversion from 50% to 60%?
(g) Plot the
rate of reaction and conversion as a function of PFR volume.
(h)
Give a critique of the answers to this problem.