Someone near you said:
I looked at your profile and I'm very interested in learning more. So tell me...how does the Biginelli Condensation work?
You replied:
The Biginelli condensation is an acid-catalyzed reaction of a beta-ketoester, an aryl aldehyde, and urea, to form 3,4-dihydropyrimidin-2(1H)-ones, which have potential for pharmaceutical application.
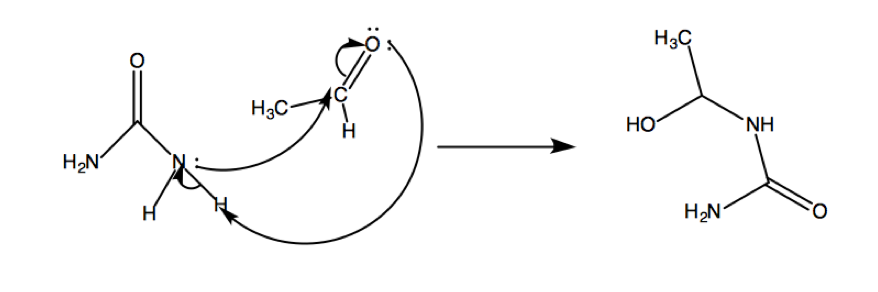
The first step in the mechanism proposed by Kappe is the nucleophilic addition by the urea to the aldehyde.

The catalytic addition of acid results in an iminium intermediate, which then acts as an electrophile and adds to the beta-ketoester.
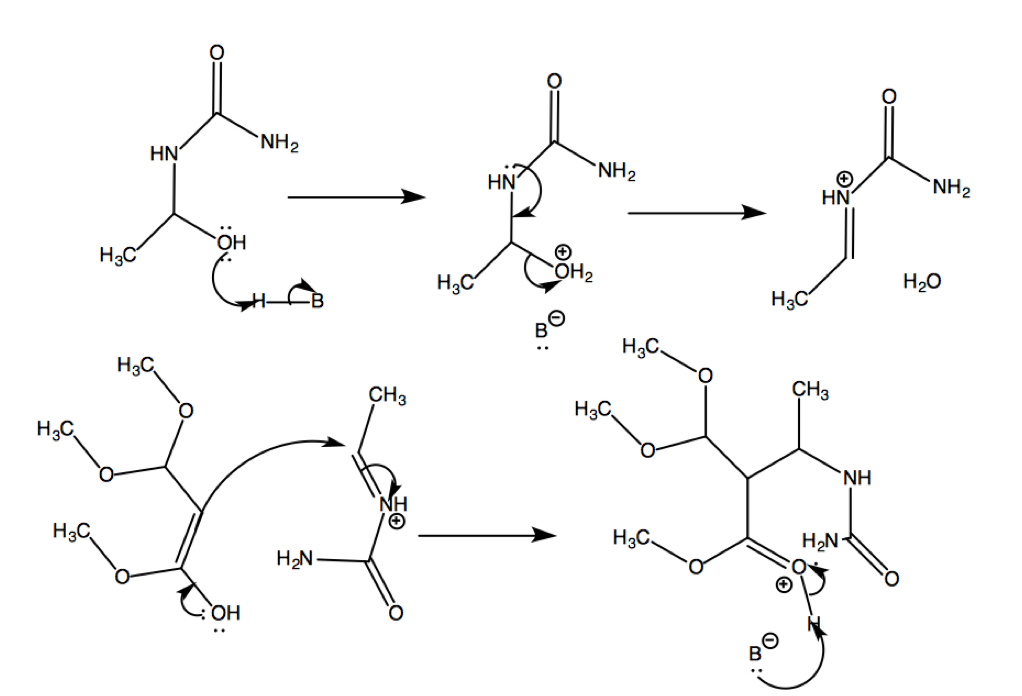
Lastly, the ketone of this resulting step undergoes condensation with the NH2 of the urea to give close the ring and complete the condensation.
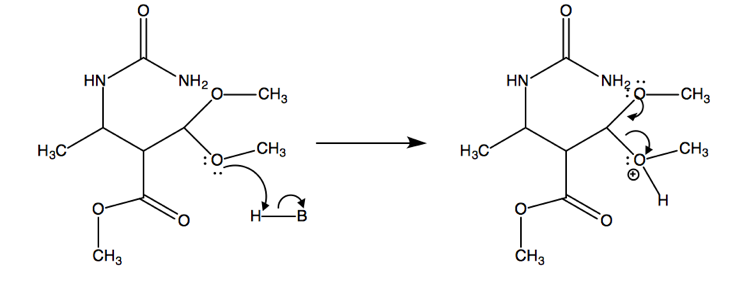
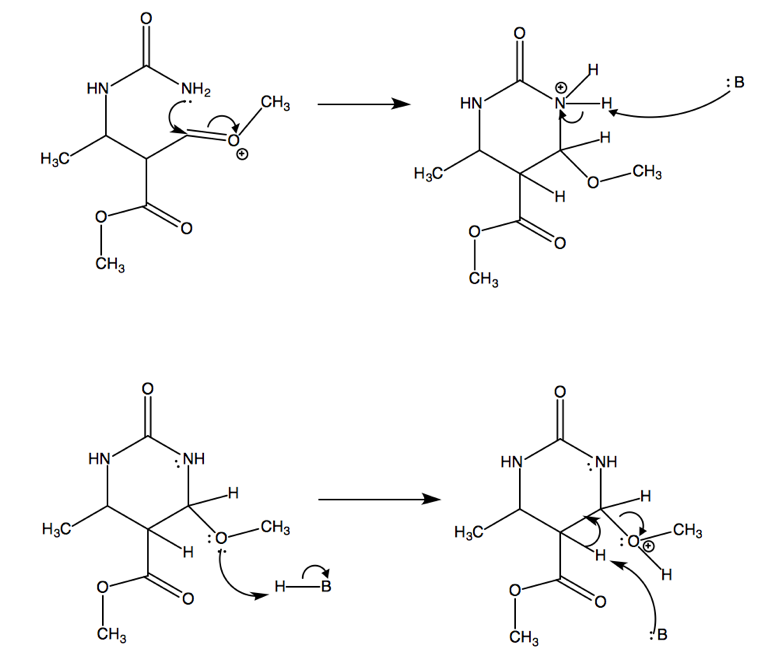
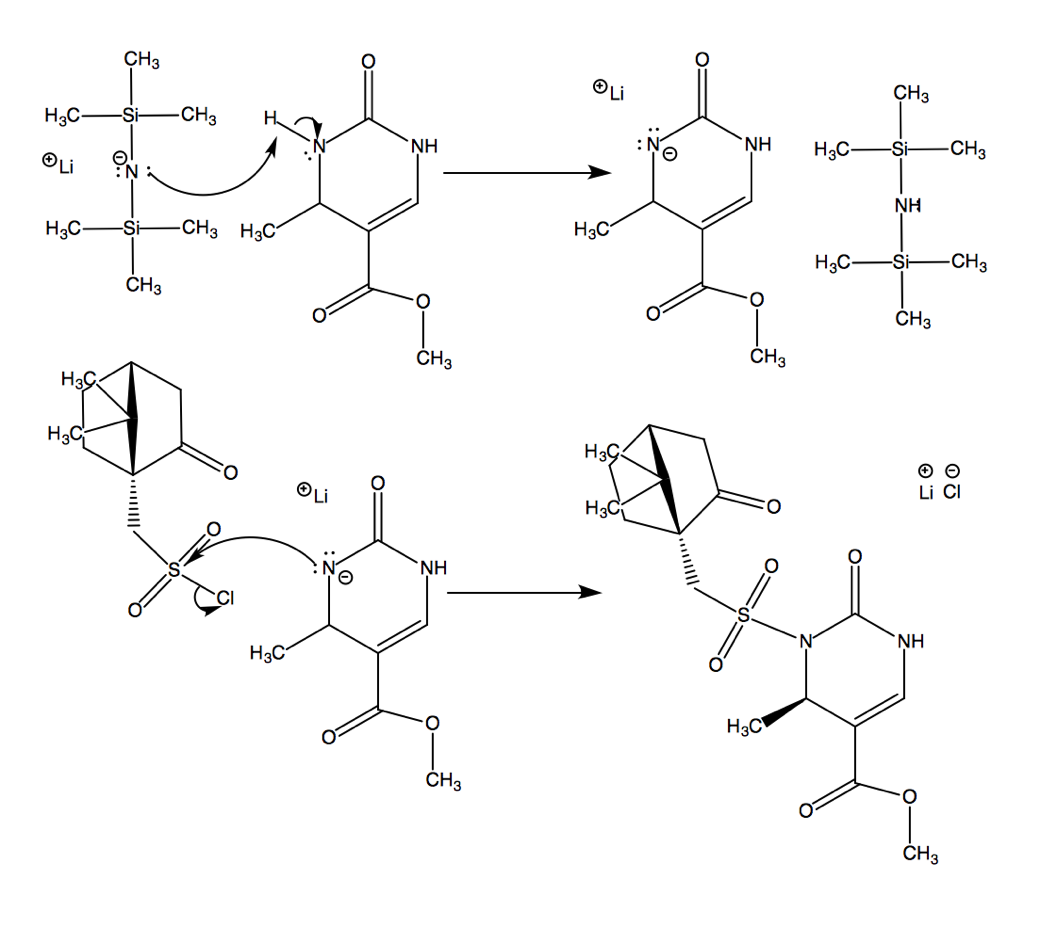
After the Biginelli condensation, this molecule goes on to perform an SN2 reaction, resulting in molecule 10a.
Evans, P. A.; Qin, J.; Robinson, J. E.; Bazin, B. Angew. Chem. Int. Ed. 2007, 46, 7417-7419.